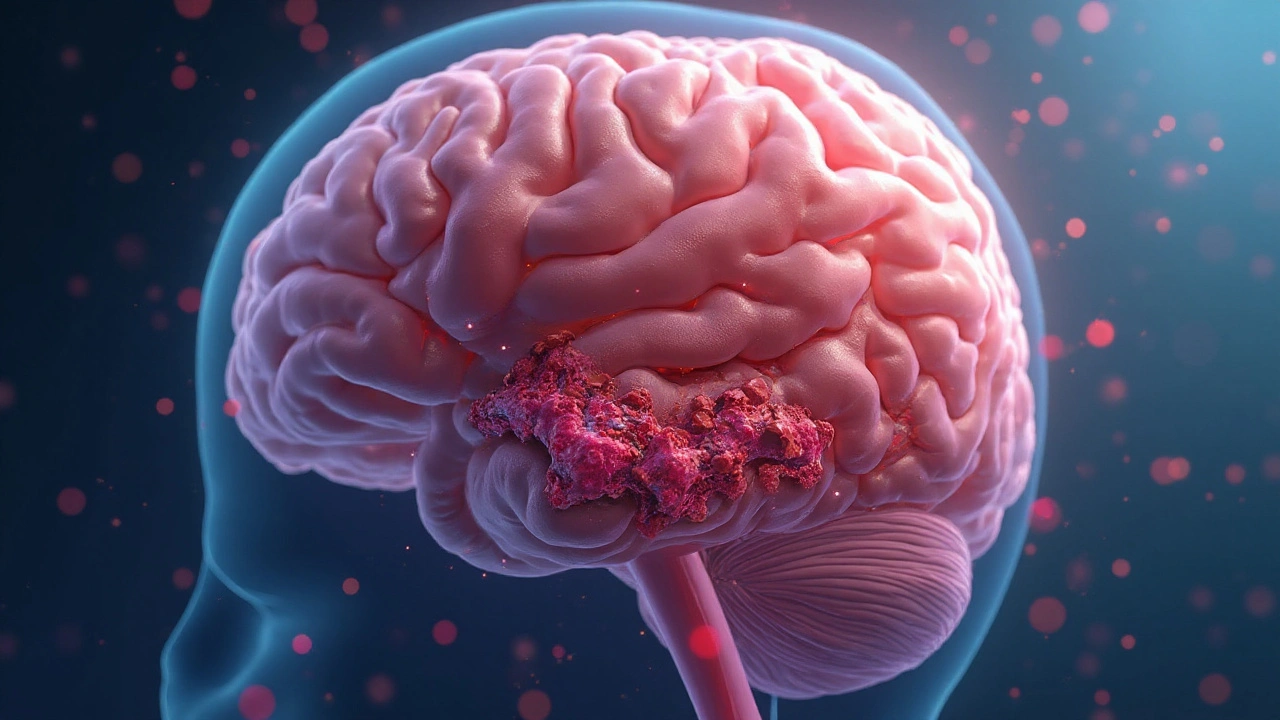
Blood clot is a gelatinous mass of platelets, fibrin, and trapped blood cells that forms to stop bleeding but can obstruct blood flow when misplaced. When a clot lodges in a cerebral artery, it can spark an ischemic stroke, the most common stroke type worldwide.
Why Blood Clots Matter for Stroke
About 87% of all strokes are ischemic, meaning a clot blocks the brain’s blood supply. The blockage starves brain tissue of oxygen and glucose, triggering cell death within minutes. In contrast, a hemorrhagic stroke results from a ruptured vessel and is less directly linked to clots, though clotting disorders can increase bleeding risk.
How Clots Form and Travel
Clot formation, or thrombosis, starts when blood vessels are damaged, blood flow slows, or the blood’s clotting cascade is over‑active. Three classic contributors-known as Virchow’s triad-drive this process:
- Endothelial injury (e.g., from hypertension or atherosclerosis).
- Stasis of blood flow (common in atrial fibrillation or prolonged immobility).
- Hypercoagulability (genetic mutations like Factor V Leiden or high platelet counts).
When a clot detaches, it becomes an embolus. The embolus rides the bloodstream, and if it reaches the brain, it can lodge in a narrow artery, producing an ischemic stroke.
Key Stroke Types Linked to Clots
Not every clot causes the same stroke. The two main clot‑related categories are:
| Attribute | Ischemic Stroke | Hemorrhagic Stroke |
|---|---|---|
| Primary cause | Clot blockage (thrombus or embolus) | Ruptured blood vessel |
| Percent of all strokes | ≈87% | ≈13% |
| Immediate treatment | Clot‑busting drugs (tPA) or mechanical removal | Surgical evacuation or blood pressure control |
| Typical outcome | Variable; early reperfusion improves survival | Higher early mortality, but survivors may have less disability |
Warning Signs: Spotting a Stroke Fast
Time is brain. Recognize the FAST acronym:
- Face drooping
- Arm weakness
- Speech difficulty
- Time to call emergency services
When a clot blocks the middle cerebral artery, symptoms often appear suddenly and affect one side of the body. Other clues include sudden vision loss, severe headache, or confusion.
Diagnosing a Clot‑Induced Stroke
Modern imaging pinpoints clot location within minutes. The two most common tools are:
- Computed Tomography (CT) - fast, rules out hemorrhage, can show hyperdense artery sign indicating a clot.
- Magnetic Resonance Imaging (MRI) - more sensitive for early ischemic changes and can visualize clot via MR angiography.
When imaging confirms an arterial occlusion, clinicians decide between intravenous tissue plasminogen activator (tPA) (if within a 4.5‑hour window) or mechanical thrombectomy (up to 24hours for selected patients).

Preventing Clots That Lead to Stroke
Prevention splits into two tracks: primary (stopping a first clot) and secondary (preventing recurrence).
Lifestyle Tweaks
- Control blood pressure - the single biggest modifiable risk factor.
- Quit smoking - reduces platelet activation.
- Maintain healthy weight and exercise - improves blood flow.
- Limit excessive alcohol - high intake can raise clotting factor levels.
Medical Strategies
When lifestyle alone isn’t enough, doctors turn to anticoagulant therapy. Options include:
- Warfarin - vitaminK antagonist, monitored via INR.
- Direct oral anticoagulants (DOACs) such as apixaban, rivaroxaban - predictable dosing, fewer food interactions.
- Platelet inhibitors (aspirin, clopidogrel) - mainly for atherosclerotic plaque‑related clots.
Patients with atrial fibrillation often receive anticoagulants because irregular heartbeats cause blood stasis in the atria, a breeding ground for clots.
Managing High‑Risk Conditions
Specific diseases amplify clot formation:
- Diabetes - elevates fibrinogen and promotes endothelial damage.
- High cholesterol - leads to plaque rupture, exposing tissue factor.
- Peripheral artery disease - signals systemic atherosclerosis, often co‑exists with cerebral artery narrowing.
Regular screening for these conditions, followed by appropriate medication (statins, antihyperglycemics), cuts clot risk substantially.
Recovery After a Clot‑Related Stroke
Even with rapid treatment, some brain tissue may be lost. Rehabilitation focuses on regaining function, relearning speech, and preventing future clots. Key components include:
- Physical therapy - restores strength and balance.
- Occupational therapy - teaches adaptive techniques for daily tasks.
- Speech‑language pathology - addresses aphasia and swallowing difficulties.
- Secondary prevention - continued anticoagulation, lifestyle adherence, and regular follow‑up imaging.
Patients who stick to a secondary prevention plan lower their recurrence risk from about 10% per year to under 3%.
Related Concepts and Next Steps
Understanding the clot‑stroke link opens doors to deeper topics such as:
- Genetic clotting disorders (e.g., Factor V Leiden, prothrombin gene mutation).
- Advanced imaging techniques like CT perfusion and diffusion‑weighted MRI.
- Emerging anticoagulants under clinical trial.
- Stroke telestroke networks that bring expert care to remote hospitals.
Readers curious about the genetic side should explore “Inherited thrombophilia and stroke risk.” Those interested in treatment tech might look up “Mechanical thrombectomy devices and outcomes.”
Frequently Asked Questions
Can a blood clot form in the brain without causing a stroke?
Yes. Small clots can lodge in tiny vessels and cause a transient ischemic attack (TIA), which resolves within 24 hours and may leave no permanent damage. However, TIAs are warning signs that a larger stroke could follow.
What is the time window for using tPA after a clot‑related stroke?
Intravenous tPA is approved for use within 4.5 hours of symptom onset. Early administration dramatically improves outcomes; each minute saved can rescue roughly 1.9 million neurons.
When is mechanical thrombectomy preferred over tPA?
Mechanical thrombectomy is considered when a large‑vessel occlusion is identified, especially if the patient is beyond the 4.5‑hour window but within 24 hours, or when tPA is contraindicated (e.g., recent surgery, bleeding risk).
How do anticoagulants differ from antiplatelet drugs in preventing stroke?
Anticoagulants (warfarin, DOACs) inhibit the clotting cascade and are optimal for preventing clots formed in the heart, such as those from atrial fibrillation. Antiplatelet agents (aspirin, clopidogrel) block platelet aggregation and are useful for atherosclerotic plaque‑related clots.
What lifestyle changes most reduce clot‑related stroke risk?
Key steps are controlling blood pressure, quitting smoking, maintaining a healthy weight, exercising regularly, limiting alcohol, and eating a diet rich in fruits, vegetables, and omega‑3 fatty acids.




While the biochemical cascade of clot formation is fascinating, the real battle begins once the embolus reaches the cerebral vasculature. Anticoagulation, especially DOACs, has reshaped the prophylactic landscape, yet adherence remains a stubborn obstacle. Moreover, the latency between atrial fibrillation detection and therapy initiation often determines whether a patient survives the FAST window. Ultimately, precision in risk stratification saves more neurons than any single drug ever could. 😊
Reading through the mechanisms makes my heart race-not just the clot, but the sheer urgency of acting fast. I’ve seen friends stumble through the FAST checklist, and those seconds felt like forever. Remember, a calm voice and clear instructions can turn a terrifying moment into a survivable one.
Clotting is just the tip of a larger agenda. The pharma lobby pushes anticoagulants while hiding side‑effects. Trust your own blood flow, not the boardroom.
🤔
Oh wow, another lecture on platelets! As if we didn’t already have enough drama in our arteries. Seriously though, the interplay between hypertension and endothelial injury is like a soap‑opera that ends in a tragedy if you ignore it. 🙄
They don’t want you to know that the “new” clot‑busting drugs are just a cash‑cow for Big Pharma. Cut the sugar, quit the smokes, and you’ll starve those sneaky clots out of existence. Simple as that, folks.
Great, another reminder that prevention is easier said than done-unless you enjoy living in a perpetual state of caution. In all seriousness, a modest 150‑minute walk each week can lower blood pressure enough to keep the clot‑factory at bay. Keep it up, and your brain will thank you.
Ever wonder why the guidelines keep changing? It's like they’re trying to keep us guessing while the hidden labs churn out the next “miracle” anticoagulant. Stay skeptical, stay safe.
Listen up! If you think a quick pill will save you, think again. You need a lifestyle overhaul-no excuses. Start tracking your BP, log your workouts, and hold yourself accountable. The only thing more dangerous than a clot is complacency.
That tPA window is like a ticking bomb-don't waste it.
Indeed, early reperfusion therapy improves outcomes; however, patient selection criteria must be rigorously applied to avoid hemorrhagic complications.
Totally got you-those therapy gaps are real. I’ve seen patients drop off after a week because the meds made them feel weird. Maybe setting up a reminder app could bridge that ditch.
Precisely; the FAST acronym serves as an essential mnemonic-prompt recognition and activation of emergency services dramatically reduces neuronal loss.
The influence of pharmaceutical funding on clinical guidelines is a documented concern; transparency and independent review are crucial to maintain public trust.
Ha! The drama of cholesterol spikes could rival any reality show. Still, those numbers aren’t just for show-they directly affect plaque stability and clot risk.
Understanding the pathophysiology of clot‑induced strokes requires a layered approach, beginning with the endothelial lining that acts as the vessel’s frontline defender. When hypertension exerts shear stress, it disrupts this barrier, exposing subendothelial collagen that invites platelet adhesion. Platelets then release granules, amplifying the cascade and converting fibrinogen into a stable fibrin mesh. Simultaneously, the coagulation cascade-initiated via the intrinsic or extrinsic pathway-produces thrombin, the enzyme that solidifies the mesh. In individuals with genetic predispositions such as Factor V Leiden, the natural anticoagulant pathways are compromised, accelerating clot formation. Atrial fibrillation introduces another variable: stagnant blood flow in the left atrial appendage, providing a fertile ground for thrombus development. When these clots dislodge, they become emboli, traveling through the arterial system until they encounter a caliber‑matching vessel, often the middle cerebral artery. The abrupt cessation of cerebral perfusion triggers the ischemic cascade, depleting ATP, disrupting ion gradients, and culminating in neuronal death within minutes. Early imaging, particularly CT angiography, can delineate the occlusion site, guiding clinicians toward either intravenous tPA or mechanical thrombectomy. The therapeutic window for tPA remains stringent-ideally within 4.5 hours-yet recent studies suggest selected patients may benefit beyond this period under strict imaging criteria. Mechanical thrombectomy, on the other hand, extends the viable intervention window up to 24 hours for large‑vessel occlusions, provided collateral circulation is adequate. Post‑procedure care emphasizes secondary prevention: antiplatelet agents for atherosclerotic disease or anticoagulants for cardioembolic sources, accompanied by aggressive blood pressure management. Lifestyle modifications, including dietary sodium reduction, regular aerobic exercise, and smoking cessation, reduce endothelial stress and lower recurrence risk. Regular follow‑up with vascular imaging ensures that any residual stenosis or new plaque formation is addressed promptly. In sum, a multidisciplinary strategy-spanning genetics, pharmacology, interventional radiology, and preventive medicine-offers the best chance to mitigate the devastating impact of clot‑related strokes.
While the exposition admirably traverses the molecular terrain, one might argue that the narrative could benefit from a more succinct articulation of the therapeutic decision algorithm, particularly regarding the nuanced interplay between perfusion imaging thresholds and mechanical thrombectomy candidacy. Moreover, an exploration of emerging pharmacologic agents-such as tenecteplase-would have enriched the discussion, given its favourable pharmacokinetic profile compared to alteplase. In any case, the comprehensive overview serves as a valuable primer for both clinicians and scholars alike.
Honestly, this reads like a textbook that never left the publisher’s desk. Real patients don’t care about the cascade of enzymes; they care about whether they’ll wake up without a slur on their tongue. Cut the jargon, give them actionable steps, and maybe they’ll listen.
It’s easy to dismiss the “big pharma” narrative, yet the reality lies somewhere between corporate profit motives and genuine medical advancement. Collaborative research that mandates data transparency can help bridge that gap, ensuring that new anticoagulants are both effective and safe.
Indeed, the paradox of preventive caution versus lived experience mirrors the ancient stoic dilemma: to act preemptively against unseen threats or to endure the consequences of complacency. In the realm of cerebrovascular health, the former often proves wiser.
Funny you mention the shifting guidelines-there’s even chatter about a secret committee fine‑tuning the dosage thresholds based on undisclosed biometric data. Until we see the raw numbers, staying informed and questioning the source remains our best defense.