Feb 2, 2026
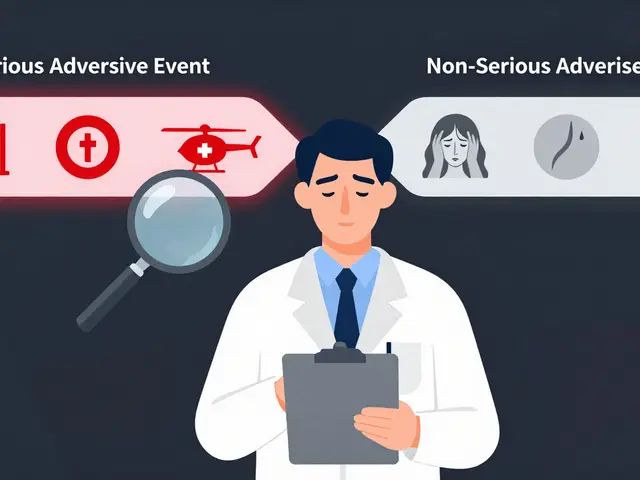
Serious vs Non-Serious Adverse Events: When to Report in Clinical Trials
Tag: serious adverse events
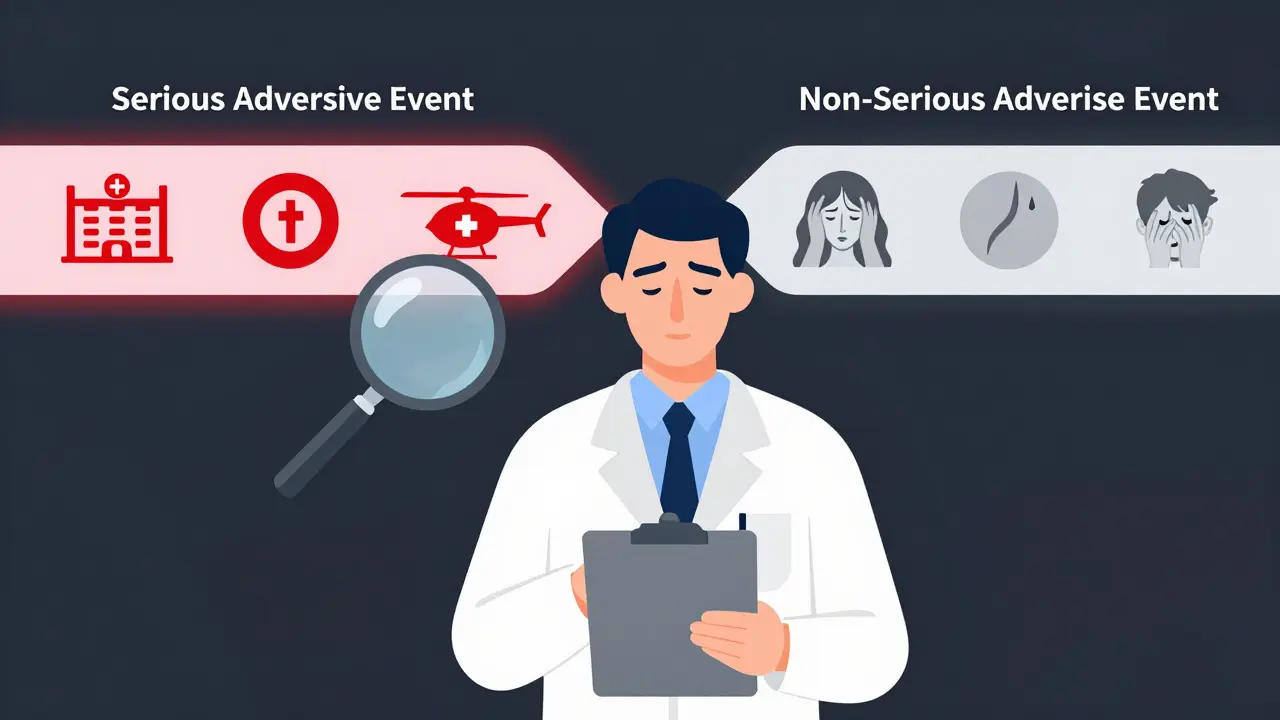
Serious vs Non-Serious Adverse Events: When to Report in Clinical Trials
Read More
Learn the critical difference between serious and non-serious adverse events in clinical trials, when to report each, and how misclassification wastes time and endangers patients. Based on FDA, ICH, and NIH guidelines.