When you're running a clinical trial, every patient reaction matters-but not every reaction needs to be reported the same way. The difference between a serious adverse event and a non-serious adverse event isn’t about how bad the symptom feels. It’s about what happens to the patient. Confusing the two can delay life-saving interventions, waste hours of staff time, and even put patients at risk.
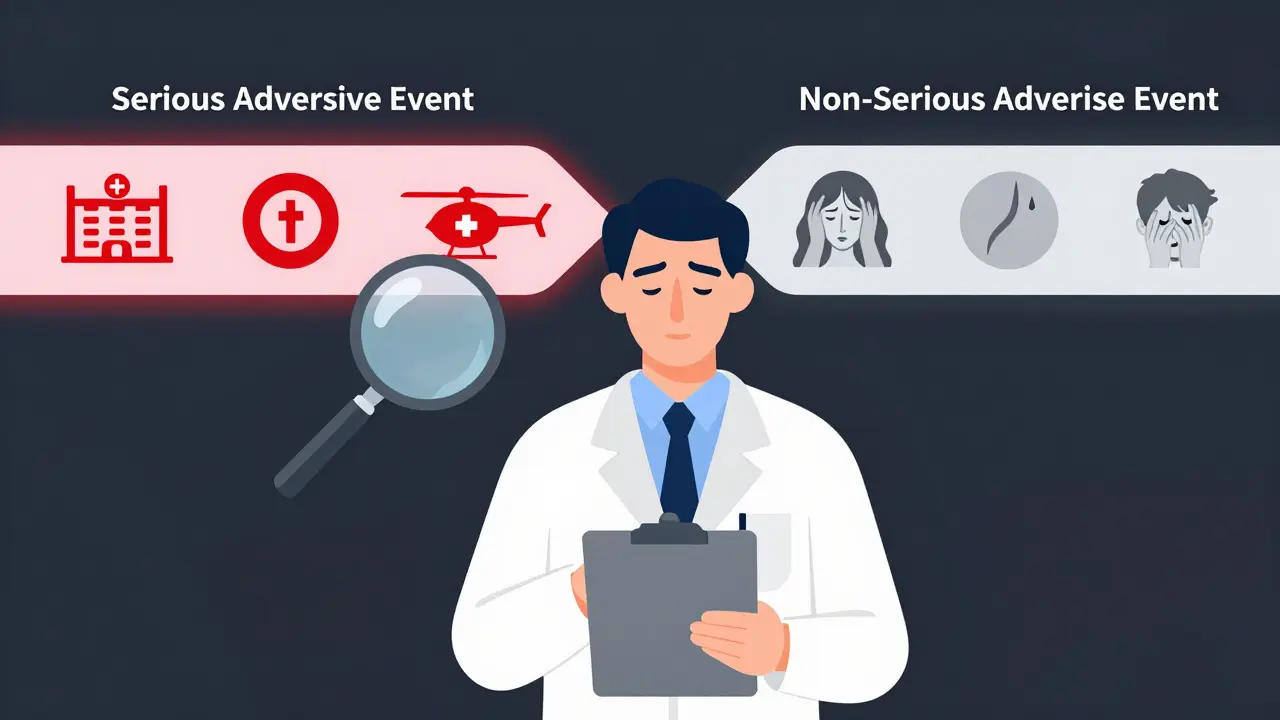
What Makes an Adverse Event 'Serious'?
An adverse event is any unwanted medical occurrence during a clinical trial, whether it’s linked to the drug or not. But only a small fraction of these are classified as serious. The FDA and ICH E2A guidelines define serious adverse events (SAEs) by six specific outcomes, not by how intense the symptoms are.
Here’s what counts as serious:
- Death
- Life-threatening condition (the patient was at immediate risk of dying)
- Requires hospitalization or extends an existing hospital stay
- Causes persistent or significant disability or incapacity
- Results in a congenital anomaly or birth defect
- Requires medical or surgical intervention to prevent one of the above
That’s it. No more, no less. A migraine that knocks someone out of work for a day? Not serious. A broken bone from a fall? Only serious if it leads to surgery or permanent damage. A rash? Unless it’s part of a life-threatening reaction like Stevens-Johnson syndrome, it’s not serious.
Here’s where people get it wrong: severity is not seriousness. A severe headache is intense-but if it goes away with ibuprofen and doesn’t lead to hospitalization, it’s not a serious adverse event. A mild rash that spreads and causes breathing trouble? That’s serious. Outcome, not intensity, decides.
When Do You Have to Report?
The clock starts ticking the moment the investigator learns about the event. For serious adverse events, the rules are strict and fast.
Investigators must report SAEs to the sponsor within 24 hours. That’s not a suggestion-it’s a legal requirement under 21 CFR 312.32. It doesn’t matter if the event seems unrelated to the drug. If it meets the seriousness criteria, it goes in immediately.
Then, the sponsor has to report to the FDA:
- 7 days for life-threatening events
- 15 days for all other serious events
For non-serious adverse events? There’s no rush. These are documented in Case Report Forms (CRFs) and reported according to the study’s Data and Safety Monitoring Plan. That could be monthly, quarterly, or bundled into routine safety updates. No 24-hour deadline. No emergency calls. Just accurate records.
IRBs (Institutional Review Boards) also need SAE reports-but with a slightly longer window. Most require them within 7 days of the investigator’s report. Non-serious events? Often not reported to the IRB at all unless the protocol says otherwise.
Why This Distinction Matters
Imagine a cancer trial. Patients come in with weak immune systems. They get fevers. They get nausea. They get rashes. If every mild symptom is reported as serious, the safety team is buried under noise. They miss the one patient who develops septic shock because they’re busy reviewing 50 reports about ‘severe fatigue’ or ‘moderate diarrhea’ that resolved on their own.
That’s not theoretical. In 2020, the European Medicines Agency found that nearly 29% of expedited safety reports didn’t meet seriousness criteria. In 2022, the University of California, San Francisco IRB spent an average of 9.7 business days per report just clarifying whether an event was serious. That’s over 100 hours lost per month just on misclassified events.
And it’s expensive. Deloitte estimated that in 2022, 62.7% of adverse event reporting costs came from processing non-serious events that were incorrectly labeled as serious. That’s hundreds of millions of dollars wasted every year.
Dr. Janet Woodcock, head of the FDA’s drug evaluation center, said it plainly: ‘The current system is overwhelmed by non-serious events reported as serious, diluting attention from truly critical safety signals.’

How to Get It Right Every Time
There’s a simple tool to avoid mistakes: the four-question decision tree from the NIH’s 2018 guidelines.
When you see an adverse event, ask:
- Did it cause death?
- Was it life-threatening?
- Did it require hospitalization or extend an existing stay?
- Did it cause persistent or significant disability or birth defects?
If the answer to any is yes → report as serious within 24 hours.
If the answer to all is no → document it as non-serious, follow your protocol’s routine reporting schedule.
Use the Common Terminology Criteria for Adverse Events (CTCAE) to grade severity (mild, moderate, severe), but don’t mix it with seriousness. Severity tells you how uncomfortable the patient was. Seriousness tells you if their life or function changed.
Most institutions now require annual training on this. The 2023 Association of Clinical Research Professionals report found that 98.7% of top research sites mandate it. If your site doesn’t, push for it. This isn’t paperwork-it’s patient safety.
What’s Changing in 2025 and Beyond
The system is getting smarter. AI tools now classify adverse events with 89.7% accuracy, compared to 76.3% for humans. The FDA is testing natural language processing to auto-triage reports-cutting review time by nearly half.
The EU’s Clinical Trials Regulation, fully active since 2022, now uses the same seriousness definition across all 27 member states. That’s cut cross-border reporting confusion by over a third.
And the ICH is rolling out E2B(R4), a global standard for electronic safety reporting, expected to be fully adopted by 2025. This will make it harder to misclassify events because systems will force you to pick the correct outcome criteria.
But tech won’t replace judgment. AI can flag a report, but a trained human still needs to confirm: Did this patient actually need to be hospitalized? Was their condition truly life-threatening? No algorithm can replace that.

Real-World Pitfalls
Some events look serious but aren’t. Here are common traps:
- Psychiatric events: ‘Severe anxiety’ sounds serious-but unless it leads to self-harm, hospitalization, or suicide attempt, it’s not an SAE.
- Emergency room visits: If someone goes to the ER for a headache and leaves without being admitted, it’s not serious. Unless they had a stroke.
- Chronic conditions: A cancer patient with worsening pain? That’s expected. Only if it’s a new, life-threatening complication (like spinal cord compression) does it become serious.
- Lab abnormalities: A high liver enzyme? Only serious if it leads to liver failure, hospitalization, or death.
And don’t forget: if you’re unsure, report it as serious. Better safe than sorry. But then follow up with your sponsor or safety team to confirm. Don’t leave it hanging.
Final Rule: Think Outcome, Not Intensity
Every time you see an adverse event, pause. Don’t think: ‘This looks bad.’ Think: ‘Did this change the patient’s life?’
If yes → report it now.
If no → record it carefully, but don’t rush.
This isn’t about bureaucracy. It’s about making sure the right people see the right signals at the right time. Misclassifying an event doesn’t just waste time-it can delay the discovery of a dangerous drug side effect. Or worse, distract from one that’s already killing people.
Get this right, and you’re not just following rules. You’re protecting lives.
Is a severe headache a serious adverse event?
No, not by itself. A severe headache is intense, but seriousness is based on outcome. Unless the headache leads to hospitalization, brain surgery, stroke, or death, it’s not a serious adverse event. It should be documented as a non-serious AE with severity graded as ‘severe’ using CTCAE.
Do I need to report an adverse event if it’s not related to the drug?
Yes. Adverse events are reported based on occurrence, not causality. Even if the event is clearly due to an unrelated condition (like a car accident or pre-existing disease), if it meets the seriousness criteria, it must be reported as an SAE. The relationship to the drug is assessed later by the sponsor.
What if I’m not sure whether an event is serious?
When in doubt, report it as serious. Use the four-question decision tree: death, life-threatening, hospitalization, or disability? If any answer is yes, report immediately. Then follow up with your sponsor or safety officer to confirm. It’s better to over-report than miss a true safety signal.
Can a non-serious adverse event become serious later?
Yes. An event initially classified as non-serious can become serious if the outcome changes. For example, a mild rash that later develops into toxic epidermal necrolysis. If new information emerges that meets the seriousness criteria, you must update the report immediately as a follow-up SAE.
Are there tools to help classify adverse events correctly?
Yes. Many sites use electronic data capture systems with built-in seriousness checklists based on FDA and ICH criteria. The FDA’s MedWatch Form 3500A includes checkboxes for each seriousness criterion. AI tools now help classify events with over 89% accuracy, but human review is still required for final determination. Training on CTCAE for severity and ICH E2A for seriousness is mandatory at most institutions.




I’ve seen this happen too many times. Someone gets a rash, logs it as an SAE, and suddenly the whole safety team is in panic mode. Meanwhile, the real red flags? Ignored. 😒 People think ‘severe’ means ‘serious’-nope. It’s not about how much it hurts. It’s about whether they’re alive at the end of the day. 🤷♂️