Foreign food factories don’t get warning before FDA shows up - and that’s the new rule
For decades, if you ran a food plant in India, China, or Mexico that shipped products to the U.S., you got a heads-up before the FDA came. A call. A letter. Maybe even a scheduled date. You had time to clean up, gather documents, hire translators, and make sure everything looked perfect. That’s over.
Starting in 2024, the U.S. Food and Drug Administration began doing unannounced inspections at foreign food facilities - just like they’ve always done at American plants. No notice. No prep time. No exceptions. If you export food to the U.S., you’re expected to be ready every single day.
This isn’t just a policy tweak. It’s a full reset of how the FDA enforces food safety across the globe. The agency now treats foreign facilities the same as domestic ones - no double standard. And the stakes are high: 15% of everything Americans eat comes from overseas. That’s millions of tons of food, every year, from over 300,000 registered facilities. One contaminated shipment can trigger recalls, border blocks, or even criminal charges.
Why the FDA changed the rules - and why it matters
The shift didn’t happen overnight. It started with a simple question: Why should foreign plants get a heads-up when U.S. plants don’t?
Before 2024, foreign facilities were routinely given advance notice so they could arrange travel, translation, and logistics. But that meant inspectors often saw a sanitized version of the facility - not the real one. A plant might have perfect records on inspection day, but what about the other 364 days? What if the contamination happened the day before, and they cleaned it up after getting the notice?
Then came the data. ProPublica reported that during the Trump administration, FDA inspections of foreign facilities dropped to historic lows. Meanwhile, imports kept rising. By 2023, 32% of fresh produce in the U.S. came from abroad. The FDA realized it couldn’t keep up - not with the old system.
So in May 2024, FDA Commissioner Martin A. Makary announced the change: “We’re ending a double standard.” Foreign plants would now be inspected the same way as U.S. ones: randomly, without warning, and with full access to records, equipment, and production lines.
It’s not just about fairness. It’s about safety. Foodborne illnesses linked to imported products cost the U.S. over $15 billion annually. The FDA’s goal isn’t to shut down foreign plants - it’s to make sure they’re safe before food hits U.S. shelves.
What the FDA looks for during an unannounced inspection
If you’re a foreign facility, you need to know exactly what the FDA is checking. It’s not just about cleanliness. It’s about systems - and whether they work every day, not just on inspection day.
- Current Good Manufacturing Practices (cGMP): Are your employees washing hands? Are surfaces cleaned between batches? Are allergens separated? These aren’t optional. They’re written into 21 CFR 117.
- Record accessibility: Your logs - temperature controls, sanitation schedules, supplier approvals - must be instantly available. Digital systems are preferred. Paper files? They better be organized and up to date.
- Facility conditions: No hiding dirty floors, broken equipment, or pest infestations. FDA inspectors can walk anywhere, take photos, and observe production in real time.
- Compliance history: If your products were ever refused entry into the U.S., you’re now on a watchlist. High-risk facilities get inspected more often.
- Language and communication: If you can’t understand an inspector’s questions, you’re at risk. The FDA doesn’t provide translators. You must have bilingual staff on-site.
The FDA doesn’t just check what’s on paper. They check what’s happening right now. One inspector might watch a worker fill a container, then ask to see the water test results from that same batch. If the records don’t match the process - that’s a violation.

What happens if you refuse or delay an inspection
Refusing an FDA inspection isn’t like ignoring a parking ticket. It’s a federal crime.
Under Section 306 of the FDA Food Safety Modernization Act (FSMA), if a foreign facility denies, delays, or limits an inspection, the FDA can immediately refuse entry of all products from that facility. That means your shipments get turned away at the border - and you lose money, time, and trust.
But it gets worse. The FDA can refer your case to the U.S. Department of Justice. You could face criminal charges. Penalties include:
- Fines up to $1 million per violation
- Forfeiture of equipment or products
- Restitution payments to affected parties
- Debarment from exporting to the U.S. for years
What counts as a violation? Examples from recent enforcement actions:
- Redacting parts of records before handing them over
- Turning off cameras or lights during inspection
- Locking doors to production areas
- Asking inspectors to pay for their own hotel or taxi
The FDA now bans inspectors from accepting any travel perks from regulated companies. No free rides. No free rooms. No gifts. This is to prevent any appearance of bias - and to make sure inspections are truly independent.
How foreign plants are adapting - and where they’re failing
Some facilities are ready. Others are in crisis.
Large manufacturers in countries like Germany, Japan, and Brazil have invested in 24/7 quality teams, digital record systems, and permanent bilingual staff. They run monthly mock inspections. They train every employee - from the warehouse worker to the CEO - on what to do if FDA shows up.
Small and medium-sized plants, especially in Southeast Asia and Latin America, are struggling. Many still rely on part-time quality managers who only show up during scheduled inspections. They keep paper records in filing cabinets. They hire translators only when they know an inspector is coming.
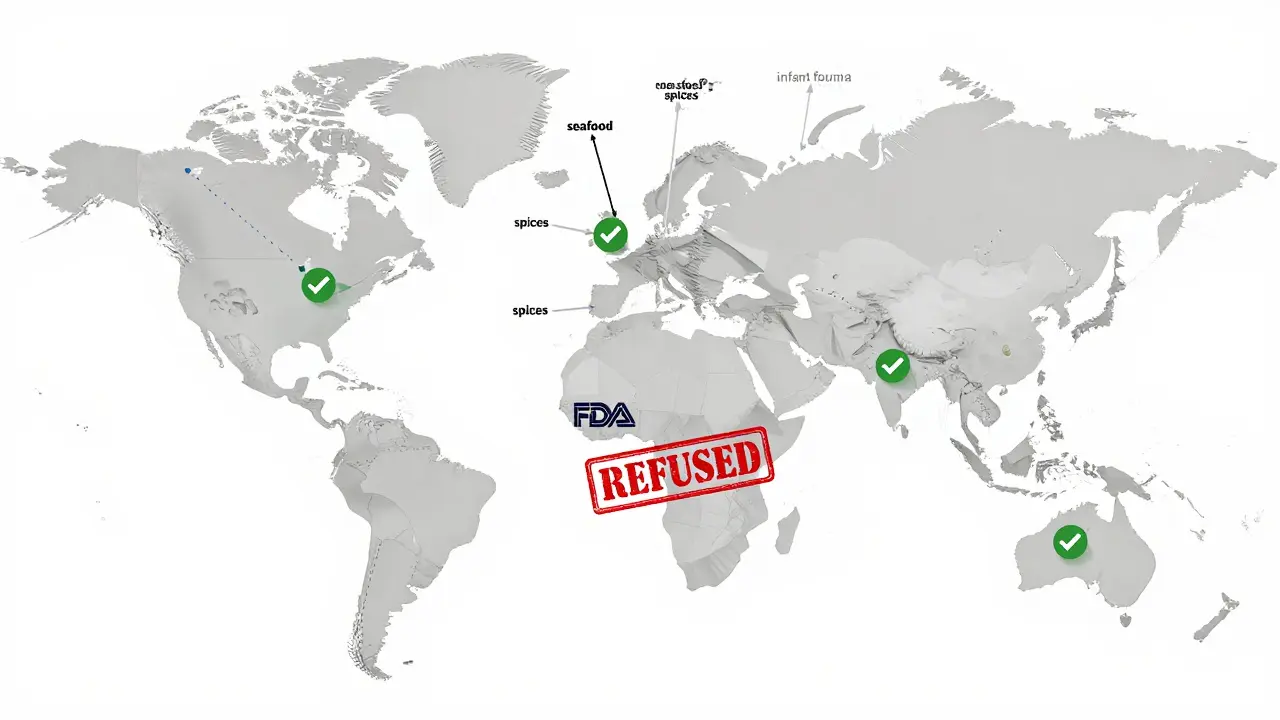
The result? A growing two-tier system. High-value exports - like organic spices, infant formula, or premium seafood - are getting inspected more often and are better prepared. Low-margin, bulk commodities - like rice, canned vegetables, or basic supplements - are being flagged more frequently for refusal.
One facility in Vietnam told an industry consultant: “We used to have two weeks to get ready. Now we have to be ready every day. We lost three shipments last year. We’re not sure we can survive another.”
What you must do right now to stay compliant
If you export food to the U.S., here’s your checklist - no exceptions.
- Digitize your records. Use cloud-based systems that are accessible 24/7. Paper files are a liability.
- Hire permanent bilingual staff. Not contractors. Not temporary hires. Someone who speaks English and understands FDA terminology.
- Train everyone. Front-line workers, managers, security - all of them. What to say. What not to say. How to respond if an inspector asks to see a machine log.
- Run weekly mock inspections. Have someone play FDA. Show up unannounced. Try to block access. See where your system breaks.
- Know your risk level. High-risk foods (raw sprouts, shellfish, nut butters) get inspected more. Know where you stand.
- Never refuse, delay, or limit access. Even if you think you’re “just protecting trade secrets.” The FDA doesn’t care.
There’s no grace period. No extension. The FDA doesn’t negotiate dates. If you’re registered to export to the U.S., you’ve already agreed to this. You just didn’t realize how serious it was until now.
What’s next for FDA foreign inspections
The FDA isn’t stopping here.
In 2025, they’re rolling out new risk-scoring algorithms that will automatically flag facilities based on:
- Historical refusal rates
- Complaints from U.S. consumers
- Supplier audits from third parties
- Geographic risk (certain countries have higher contamination rates)
They’re also testing third-party inspectors - certified private auditors who work under FDA oversight. This could speed up inspections and reduce backlogs.
And they’re pushing for global alignment. Other countries - Canada, the EU, Australia - are watching. If the FDA’s system works, they’ll copy it. That means what you’re doing now to comply with U.S. rules will soon be the global standard.
The message is clear: Food safety isn’t a favor the U.S. gives you. It’s a requirement. And if you want to sell to American consumers, you play by American rules - no exceptions, no warnings, no second chances.
Do I need to be ready for an FDA inspection every day?
Yes. Since May 2024, the FDA conducts unannounced inspections at foreign food facilities - just like they do in the U.S. There is no advance notice. If you export food to the United States, your facility must be in full compliance 365 days a year. That means clean facilities, accessible records, trained staff, and no last-minute cleanup.
What happens if I refuse to let FDA inspectors in?
Your products will be automatically refused entry at the U.S. border. The FDA can also refer your case to the U.S. Department of Justice for criminal prosecution. Penalties include fines up to $1 million, forfeiture of equipment, and debarment from exporting to the U.S. for years. Refusing an inspection is treated as a federal crime.
Can I hire a translator only when an inspection is expected?
No. The FDA does not provide translators, and you cannot delay an inspection to arrange one. You must have qualified bilingual staff permanently on-site who can communicate with inspectors in English at any time. Relying on temporary translators is a common reason for inspection failures.
Are digital records required for FDA inspections?
Paper records are still allowed, but they must be immediately accessible and fully up to date. Digital systems are strongly preferred because they’re easier to verify in real time. Many facilities now use cloud-based quality management software that logs everything - from temperature readings to sanitation schedules - automatically.
How often does the FDA inspect foreign facilities?
There’s no fixed schedule. Inspections are risk-based. High-risk facilities (like those producing raw meat, seafood, or infant formula) or those with past violations may be inspected every year. Lower-risk facilities might be inspected every 3-5 years - or even less. But all are subject to unannounced visits at any time.
Can I pay for the FDA inspector’s travel or hotel?
No. Since 2024, FDA inspectors are strictly prohibited from accepting any travel accommodations, meals, or gifts from foreign facilities. This includes taxis, hotels, or even coffee. Any attempt to provide these can be seen as an attempt to influence the inspection and may lead to enforcement action.
Bottom line: Compliance isn’t optional - it’s survival
If you’re exporting food to the U.S., your business is now on the FDA’s radar - and they’re watching every day. The old way of preparing for inspections is gone. The new rule is simple: Be ready. Always.
It’s not about perfection. It’s about consistency. About systems that work without notice. About people who know what to do when the door opens and someone in a blue jacket walks in with a clipboard.
The FDA isn’t trying to punish you. They’re trying to protect your customers - and your business. Because if your food makes someone sick, it’s not just a recall. It’s a brand destroyed. A market lost. A future gone.
Don’t wait for the inspector to show up. Start preparing today - because tomorrow might be the day they come.







This is huge for us in India. We’ve been cleaning up our act for months now. No more last-minute panic. 🙏 But honestly? The FDA’s zero-tolerance approach is scary. One typo in a log and we’re flagged. Still, better safe than sorry. Global standards are coming, and we’re learning to dance in the rain. 🌧️
Let’s be clear: this isn’t ‘fairness’-it’s enforcement. The FDA’s been playing whack-a-mole with import refusals since 2018. The real issue? 78% of foreign facilities still rely on paper logs and weekend quality staff. That’s not negligence-it’s negligence with a visa. cGMP isn’t a suggestion. It’s 21 CFR 117. If you can’t comply, you shouldn’t be exporting. Period.
So now we’re supposed to have bilingual staff on standby 24/7? Cool. I guess that’s why my cousin’s spice company in Kerala got their third refusal last month. They didn’t even know the inspector spoke Mandarin.
Yo, if you’re still using paper logs, you’re basically leaving your door unlocked and hoping no one steals your TV. Just get a cloud-based QMS. They’re cheap now. Run a mock inspection every week. Train your janitor. Seriously. It’s not magic-it’s just not being an idiot.
This is actually a win for global food safety. The U.S. isn’t being harsh-just consistent. In Brazil, we’ve had unannounced checks for years. You adapt. You build systems. You stop treating compliance like a seasonal task. The FDA’s just catching up to the rest of the world.
This is ridiculous. The U.S. is imposing its bureaucratic standards on sovereign nations. Why should a small Thai noodle factory be held to the same standard as a Tyson plant? We don’t inspect American factories the same way we inspect theirs. Hypocrisy is still hypocrisy, even when it’s dressed in lab coats.
I’ve been in food safety for 18 years. The real game-changer here isn’t the unannounced inspections-it’s the refusal of travel perks. That’s the first time the FDA has truly insulated itself from influence. It’s small, but it’s massive. It means the data is clean. And clean data means better risk scoring. This is the future.
Digitize records. Train everyone. No translators on call. Mock inspections weekly. That’s it. No fluff. No excuses. Do it or get off the list.
Let’s not sugarcoat this: the FDA is now a global enforcer with teeth. And frankly, most foreign facilities aren’t just unprepared-they’re structurally incapable of meeting these standards. Small plants in Vietnam, Indonesia, Pakistan-they’re not failing because they’re lazy. They’re failing because they’re trapped in a global supply chain that treats them as disposable. The FDA’s policy is technically sound. Ethically? It’s a blunt instrument. And it’s going to break a lot of small businesses before it fixes anything.
I know this sounds harsh, but if you’re exporting to the U.S., you’re already in the big leagues. This isn’t about punishment-it’s about respect. Respect for the people who eat your food. If you can’t meet the baseline, maybe you need to rethink your market. Not everyone has to be in the U.S. And that’s okay.
It’s funny how we treat safety like a checklist. But food isn’t a spreadsheet. It’s biology. It’s decay. It’s time. The FDA’s system assumes control. But control doesn’t prevent contamination-it just delays the moment it’s discovered. Maybe what we need isn’t more inspections. Maybe we need better fermentation protocols, better supply chain transparency, better trust. But I guess that’s harder to audit.