When a brand-name drug loses patent protection, patients and pharmacies expect cheaper alternatives. But not all generics are the same. Some are made by the original manufacturer - identical in every way to the brand, just with a different label. These are called authorized generics. They’re not just similar - they’re the exact same pill, capsule, or injection you got from the brand, just sold under a different name. And right now, they’re becoming harder to find.
What Exactly Is an Authorized Generic?
An authorized generic is a brand-name drug that’s sold without the brand name on the label. The company that made the original drug - say, Pfizer or AbbVie - also makes this version. It has the same active ingredients, same inactive fillers, same shape, same color, same everything. The only difference? The packaging and the name on the box.
The FDA defines it clearly: it’s a drug approved under a New Drug Application (NDA), but marketed with different labeling or product codes. That’s it. No extra testing. No bioequivalence studies. Because it’s made on the same line, by the same team, using the same formula.
This matters because traditional generics - the ones you see at Walmart or CVS - must prove they work the same way. But they can use different fillers. And sometimes, those fillers cause problems. Patients switching from brand to generic have reported side effects like dizziness, nausea, or mood changes - not because the active drug changed, but because the binder or coating did. Authorized generics avoid that entirely.
Why Are Authorized Generics So Rare Now?
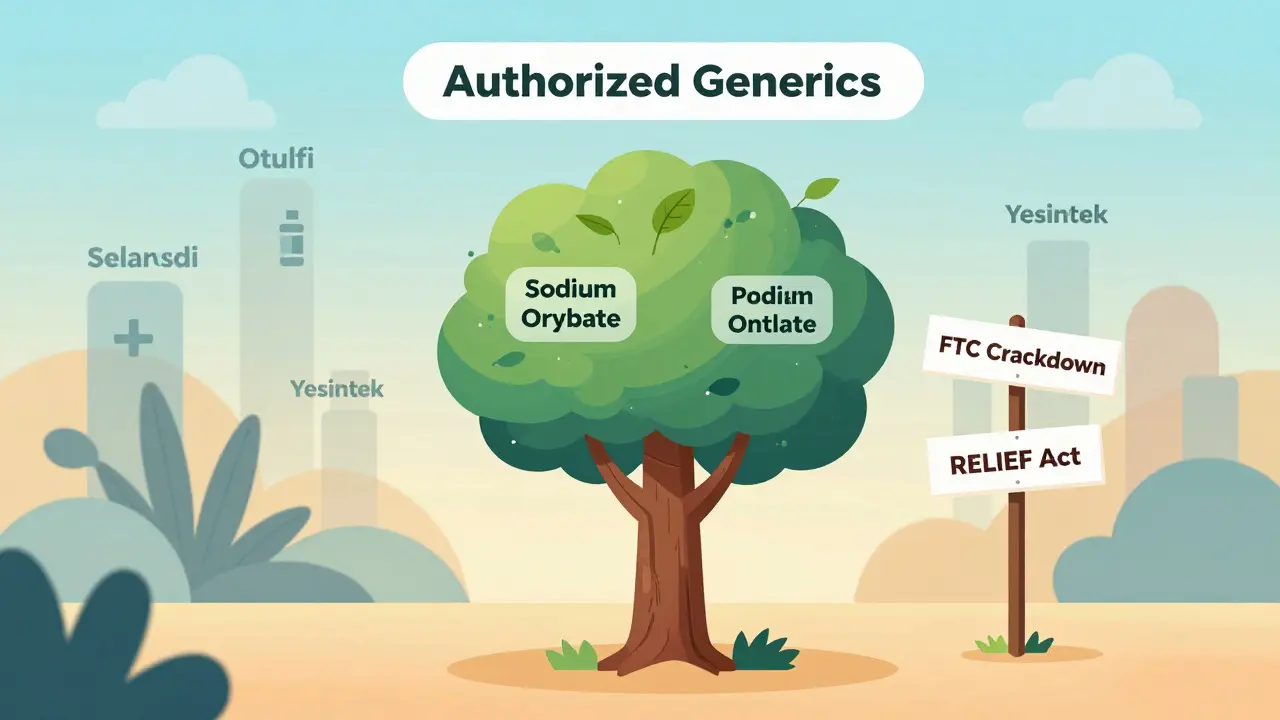
In 2022, the FDA approved 37 new authorized generics. In 2025? Only 12. That’s a 68% drop in just three years.
Why? The Federal Trade Commission (FTC) started cracking down. For years, brand companies used authorized generics as a tactic to delay real competition. They’d launch their own generic version right when the patent expired - undercutting the real generic makers, confusing pharmacies, and keeping prices high. The FTC called it a "pay-for-delay" scheme. And in 2023, they forced Teva to pay $1.2 billion for doing exactly that with Copaxone.
Since then, brand companies have pulled back. Why risk a lawsuit when you can just wait for biosimilars? Especially for biologics - complex drugs like Humira or Stelara - the path to market is clearer now. In 2025 alone, five interchangeable biosimilars to Stelara were approved. None of them were authorized generics. Why? Because you can’t make an authorized generic of a biologic. They’re too complex. So companies are shifting strategy.
What Authorized Generics Are Available Right Now?
As of October 2025, the FDA lists 1,247 authorized generic products. Most are in three areas: heart medications, brain drugs, and diabetes treatments.
Two new ones were added in late 2025:
- Sodium oxybate (Xyrem) - now available as an authorized generic from Jazz Pharmaceuticals. Used for narcolepsy and cataplexy.
- Plecanatide (Trulance) - now sold under a different label by Ironwood Pharmaceuticals. Treats chronic idiopathic constipation and IBS-C.
These are the only two new authorized generics approved in 2025. That’s it.
Don’t confuse them with regular generics. For example, in 2025, multiple versions of denosumab (used for osteoporosis and bone cancer) hit the market: Ospomyv, Xbryk, Bildyos, Aukelso, Enoby. These are traditional generics - different manufacturers, different inactive ingredients. Same drug, different pills. Not authorized generics.
Same with ustekinumab. Otulfi, Selarsdi, Yesintek - these are biosimilars to Stelara. Not authorized generics. They’re not made by AbbVie. They’re made by other companies using different processes.
Are They Cheaper? The Price Puzzle
Here’s the catch: authorized generics aren’t always cheap.
Traditional generics often cost 80% less than the brand. Authorized generics? Usually just 10% to 15% cheaper. Why? Because the brand company still controls the supply. They’re not trying to win on price - they’re trying to win on trust.
Patients who’ve switched from brand to authorized generic often report no change in how they feel. A 2024 JAMA study found doctors trust them more. Patients on the authorized generic of Lyrica (pregabalin) were 68% more likely to say it worked exactly like the brand, compared to 52% for regular generics.
But insurance doesn’t always care. Blue Cross and other insurers have started removing authorized generics from their formularies. Why? Because their pharmacy benefit managers (PBMs) got better deals on traditional generics. Even if the authorized generic is identical, the rebate is smaller. So the insurer pushes the cheaper (but chemically different) version.
One pharmacist on Reddit shared a patient story: a woman stabilized on the authorized generic of sertraline had a panic attack after her pharmacy switched her to a regular generic. The fillers triggered anxiety. She had to go back to the authorized version - and pay $120 a month instead of $15.
Why Pharmacists Struggle to Identify Them
Most pharmacists can’t tell the difference between an authorized generic and a regular generic just by looking at the bottle.
A 2025 survey of 2,345 pharmacists found 63% couldn’t reliably identify authorized generics without checking the FDA’s Orange Book. That means patients are getting counseling they don’t need - or worse, getting switched to a different version without knowing.
The FDA doesn’t require special labeling. No "Authorized Generic" stamp. No bright yellow sticker. Just a different NDC code. If your pharmacy’s system doesn’t flag it, you won’t know.
That’s why some schools, like the Community College of Philadelphia, now teach pharmacy techs how to spot them. It’s becoming a necessary skill.
What’s Next for Authorized Generics?
The future looks dim. Evaluate Pharma predicts authorized generics will make up less than 5% of all generic drug entries by 2027.
Why? Two big reasons:
- The FTC is still watching. Any move that looks like anti-competitive behavior gets flagged fast.
- The RELIEF Act (H.R. 4086), introduced in May 2025, would force authorized generics to match the price of traditional generics. That kills the business model. Why make an authorized generic if you can’t charge more than the competition?
But there’s still a place for them - especially for drugs where tiny differences matter. Think epilepsy meds, blood thinners, thyroid pills. For these, the identical formula of an authorized generic can mean fewer seizures, fewer clots, fewer crashes.
Dr. Aaron Kesselheim at Harvard calls them an "underutilized patient safety tool." And he’s right. When you’re on a narrow therapeutic index drug, identical matters. Not just similar. Identical.
What Should You Do?
If you’re on a brand-name drug that’s going generic:
- Ask your doctor: "Is there an authorized generic for this?"
- Ask your pharmacist: "Is this the same pill as the brand?"
- Check the FDA’s Authorized Generics list - it’s public and updated quarterly.
- If your insurance denies coverage for the authorized version, appeal. Show them it’s identical. Sometimes it works.
Don’t assume all generics are equal. Some are. Some aren’t. And sometimes, paying a little more for the exact same drug is worth it.
Are authorized generics the same as brand-name drugs?
Yes. Authorized generics are chemically and physically identical to the brand-name drug. They’re made on the same production line, with the same ingredients, by the same company. The only difference is the label and packaging.
Why are authorized generics more expensive than regular generics?
Because the brand company still controls them. They don’t need to compete on price - they’re already the original product. Regular generics are made by competitors who slash prices to win market share. Authorized generics usually cost only 10-15% less than the brand, while regular generics can be 80% cheaper.
How do I know if my prescription is an authorized generic?
Look up the drug’s NDC code on the FDA’s Authorized Generics list. You can also ask your pharmacist directly. Most pharmacies don’t label them as such, so you’ll need to check. Don’t rely on the name on the bottle - it’s often misleading.
Can I switch from a brand to an authorized generic safely?
Yes, and it’s often safer than switching to a regular generic. Since the formulation is identical, there’s no risk of side effects from different fillers or coatings. This is especially important for drugs with narrow therapeutic indexes, like seizure medications or blood thinners.
Why are there so few authorized generics now?
The FTC cracked down on brand companies using them to block real competition. In 2022, 37 were approved. In 2025, only 12. Plus, new laws like the RELIEF Act could force them to match traditional generic prices, making them unprofitable. Companies are shifting to biosimilars instead.




So let me get this straight - the same damn pill, same factory, same chemists, just a different label, and suddenly it’s ‘cheaper’? Nah. It’s just the brand playing 4D chess while we’re stuck playing checkers. You pay 10% less for the exact same thing? That’s not a generic. That’s a tax on gullibility. Meanwhile, real generics get slammed for ‘not being identical’ - but only when they’re not made by the same company. Hypocrisy wrapped in a pill bottle.
I just wanted to say thank you for this post. I’ve been on sertraline for years and switched to the authorized generic last year - no issues at all. My anxiety stayed gone, my sleep improved, and I saved like $100 a month. It’s not magic, it’s just science. Don’t let anyone tell you otherwise. You deserve to feel stable without getting broke. 💙
They’re lying. All of it. The FDA? In bed with Big Pharma. The FTC? A joke. You think they really care about you? Nah. They want you on the cheap generics so they can track your data, sell your health info, and upsell you on supplements. That ‘authorized’ label? It’s a trap. They’re testing side effects on you. Look at the NDC codes - they’re all linked to the same server farm in Virginia. I’ve seen the leaks.