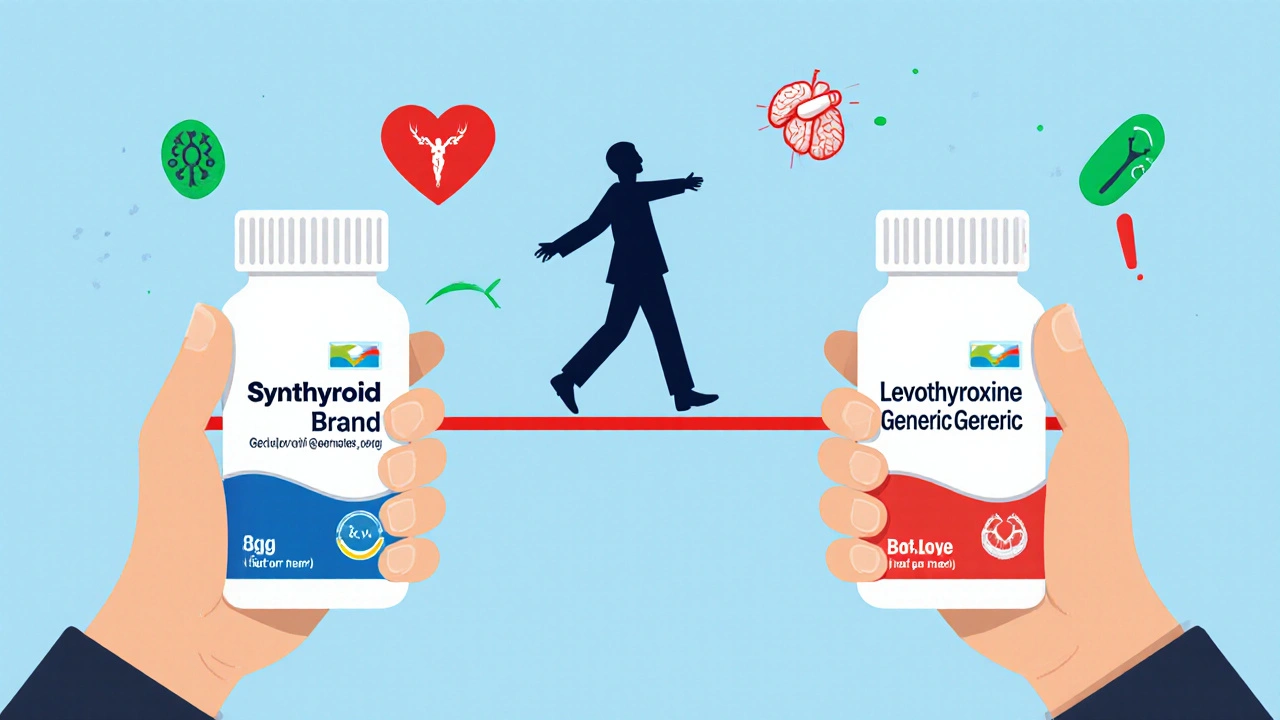
When your doctor prescribes a medication like levothyroxine, warfarin, or tacrolimus, you might not realize you’re taking a NTI drug. These aren’t your everyday pills. A narrow therapeutic index (NTI) drug means the difference between working perfectly and causing serious harm is tiny. Too little? The condition comes back. Too much? You could end up in the hospital. That’s why switching from a brand-name version to a generic isn’t just a cost decision-it’s a clinical one.
What Makes an NTI Drug Different?
NTI drugs have a razor-thin window between the dose that treats your condition and the dose that harms you. The FDA defines them as medications where small changes in blood concentration can lead to treatment failure or life-threatening side effects. Think of it like walking a tightrope-step too far left or right, and you fall.
Common NTI drugs include:
- Levothyroxine (for hypothyroidism)
- Warfarin (a blood thinner)
- Tacrolimus (used after organ transplants)
- Phenytoin and carbamazepine (for seizures)
- Cyclosporine (another immunosuppressant)
These aren’t rare. Levothyroxine alone is prescribed to over 20 million Americans. Warfarin is one of the most common anticoagulants in the world. And yet, most people don’t know they’re on an NTI drug until they’re told to stick with the brand or warned about switching.
Why Do Generics Even Exist for NTI Drugs?
Generic drugs are cheaper-often 30% to 85% cheaper. A month’s supply of brand-name Synthroid can cost $30-$60. The generic version? As low as $4. That’s not just savings-it’s access. For people on fixed incomes, that difference means choosing between medication and groceries.
The FDA requires all generics to prove they’re bioequivalent to the brand. For most drugs, that means the generic’s blood levels must fall within 80-125% of the brand’s. But for NTI drugs, the rules changed in 2014. Now, the acceptable range can be as tight as 90-111%, depending on how the original drug behaves in the body. That’s stricter than most people realize.
The FDA’s Orange Book lists generics with an “AB” rating, meaning they’re considered interchangeable. But here’s the catch: just because a generic is rated AB doesn’t mean every batch behaves the same way across different manufacturers. That’s where things get messy.
Is It Safe to Switch? The Evidence Says… It Depends
A 2022 FDA study tracked nearly 18,000 patients on levothyroxine. It found no significant difference in thyroid hormone levels between those on brand and generic versions. That’s strong evidence that, for many, switching is fine.
Another analysis of 3.5 million patients across chronic conditions-including diabetes, high blood pressure, and depression-showed no major drop in outcomes when generics were used. That’s reassuring.
But then you look at tacrolimus. Studies show that switching between generic versions-even ones with the same “AB” rating-can cause dangerous spikes or drops in blood levels. Transplant patients need stable levels to avoid rejection or toxicity. One study found that after switching manufacturers, 1 in 5 patients needed a dose adjustment within weeks.
And then there’s phenytoin. A 2022 FDA advisory committee pointed out that even tiny changes in blood concentration can trigger seizures in patients who’ve been stable for years. That’s why some neurologists still insist on keeping patients on the same brand-or even the same generic manufacturer.
The data isn’t black and white. For some NTI drugs, generics work just fine. For others, the risk isn’t worth it.
What Do Real Patients Experience?
Online forums tell a different story than clinical studies. On Reddit’s r/Thyroid, users report everything from smooth transitions to sudden fatigue, weight gain, and heart palpitations after switching to a generic levothyroxine. One user wrote: “I was fine on Synthroid for 8 years. Switched to generic. Three weeks later, my TSH jumped from 2.1 to 8.9. I had to go back to brand.”
On PatientsLikeMe, warfarin users report similar patterns. About 78% had no issues. But 22% saw their INR (a measure of blood clotting) swing unpredictably. Some needed weekly blood tests for months to get back on track.
For epilepsy patients, the Epilepsy Foundation’s 2022 survey found 42% reported breakthrough seizures after switching generics. That number might be inflated because it’s self-reported. But it’s not ignored. Neurologists still see cases where a switch triggered seizures that hadn’t occurred in years.
These aren’t outliers. They’re signals. When a drug has a narrow window, your body’s response matters more than the label.
What Should You Do?
Here’s the practical truth: NTI drugs aren’t one-size-fits-all. Your best move isn’t to blindly accept a generic or refuse it outright. It’s to be informed and proactive.
- If you’re starting treatment: Go with the generic. It’s safe, effective, and saves money. Most people do fine.
- If you’re already stable on brand: Talk to your doctor before switching. Don’t let a pharmacy change your prescription without your knowledge.
- If you’ve switched and feel off: Don’t wait. Get your blood levels checked. For levothyroxine, that’s TSH. For warfarin, it’s INR. For tacrolimus, it’s trough levels.
- If you’re on a generic and doing well: Stay on it. But know the manufacturer. If your pharmacy switches to a different generic, ask if it’s the same one.
Many states have laws requiring pharmacists to notify you or get your doctor’s approval before substituting an NTI drug. Check your state’s rules. In 28 states, you have legal protection against automatic substitution.

Insurance and Cost: The Hidden Pressure
Insurance companies push generics hard. Blue Cross Blue Shield of Kansas, for example, makes you pay the full price difference if you want the brand. That’s $40 extra a month. For many, that’s not an option.
But here’s the thing: if you need the brand because a generic caused problems, your doctor can write “dispense as written” on the prescription. That legally blocks substitution. You don’t need to fight it alone.
Some insurers now require prior authorization for brand-name NTI drugs. That’s not a roadblock-it’s a checkpoint. It means someone is reviewing whether the switch is safe for you.
The Future: Personalized Decisions, Not Blanket Rules
The FDA is launching a new NTI Drug Registry in 2023 to track real-world outcomes after substitutions. The AHRQ is funding a $2.4 million study tracking 50,000 patients across 15 health systems. By 2025, we’ll have better data on which NTI drugs can safely be switched-and which ones shouldn’t be touched.
One thing is clear: blanket bans or blanket approvals don’t work. The answer isn’t “always brand” or “always generic.” It’s “what works for you.”
Your body reacts to medication in ways no lab test can fully predict. That’s why consistency matters. Once you find a version-brand or generic-that keeps you stable, stick with it. Don’t let cost or convenience override your health.
Bottom Line: Stay Alert, Not Afraid
You don’t need to fear generics. You need to be smart about them. For most NTI drugs, generics are safe and effective. But for some, even a small change can cause big problems.
Ask questions. Know your drug. Track your symptoms. Get blood tests when needed. And never assume a pharmacy change is harmless.
If you’re on an NTI drug, your best ally isn’t the cheapest option-it’s your awareness. Stay informed. Stay in control. And don’t let anyone make the decision for you.
Are generic NTI drugs as safe as brand-name versions?
For many NTI drugs, yes-generic versions are just as safe and effective. Studies on levothyroxine and warfarin show no major difference in outcomes for most patients. But for drugs like tacrolimus and phenytoin, switching between manufacturers can cause dangerous fluctuations in blood levels. The key is consistency: once you find a version that works, stick with it.
Can a pharmacy switch my NTI drug without telling me?
In 28 U.S. states, pharmacists are legally required to notify you or get your doctor’s approval before substituting an NTI drug. In other states, automatic substitution is allowed unless your prescription says “dispense as written.” Always check your prescription label and ask your pharmacist if a change was made.
What should I do if I feel worse after switching to a generic?
Don’t ignore it. Contact your doctor right away. For levothyroxine, request a TSH test. For warfarin, check your INR. For immunosuppressants like tacrolimus, ask for a blood level test. Symptoms like fatigue, dizziness, irregular heartbeat, or new seizures could mean your drug levels are off. You may need to switch back to your original version.
Why do some doctors refuse to allow generic substitution for NTI drugs?
Some doctors have seen patients experience serious side effects after switching-especially with antiepileptics or transplant medications. Even if studies show generics are generally safe, individual reactions vary. For patients who are stable, the risk of switching isn’t worth the cost savings. Doctors prioritize stability over savings when the margin for error is tiny.
Is there a list of all NTI drugs?
The FDA doesn’t publish an official public list, but clinical experts agree on about 15-20 common NTI drugs. These include levothyroxine, warfarin, tacrolimus, cyclosporine, phenytoin, carbamazepine, digoxin, and lithium. Always check with your pharmacist or doctor if you’re unsure whether your medication falls into this category.




Been on generic levothyroxine for 5 years now. TSH stable as a rock. No issues. My endo says if it ain't broke don't fix it but if you start on generic and feel fine stay there. No need to chase brand unless you have a problem.
YALL. I switched to generic warfarin last year and my INR went from 2.4 to 4.8 in 10 days. I was bleeding out my nose at 3am. Took me 3 months to stabilize. Now I only take Synthroid and Coumadin. No more generics for me. My body ain't a lab rat.
Generic is for poor people. You want to live? Stick with brand. Simple.
It's not about brand vs generic. It's about control. The pharmaceutical industry wants you to believe consistency is a myth. But your body remembers. It remembers the fillers. The binders. The microscopic differences in crystalline structure. You think your thyroid doesn't know the difference between a tablet made in India and one made in New Jersey? It does. It always does. You're not just taking a pill. You're negotiating a silent contract with your biology. And when that contract is broken? You pay the price in fatigue. In tremors. In panic attacks at 2am. The FDA doesn't measure soul-deep stability. They measure blood concentration. That's not medicine. That's accounting.
Oh sweet mercy. Another post about NTI drugs. Can we please just admit that Big Pharma and the FDA are in cahoots? They want you on generics because they make more money. They don't care if you have a seizure or your transplant fails. They have their quarterly reports to hit. And you? You're just a data point in their profit matrix. I've seen it. My cousin's kidney transplant? Switched to generic tacrolimus. Rejection within 3 weeks. Now he's on dialysis. And the pharmacy? They just sent him another script. Same generic. Different batch. Same disaster waiting to happen.
Let me guess. This is another liberal propaganda piece disguised as medical advice. The FDA is a puppet of the WHO. The WHO is controlled by the UN. The UN is funded by George Soros. And now they want to force generic NTI drugs on Americans to destabilize our healthcare system and reduce our population. You think your TSH levels are your own? They're being monitored. They're being recorded. They're being used to determine your eligibility for future medical subsidies. Stay on brand. Stay free.
generic levothyroxine gave me anxiety so bad i cried in the shower every day for 2 weeks. switched back to synthroid and boom. normal. also the generic tasted weird. like metal. why does that even matter? it does. my body knows.
It is an undeniable truth that the substitution of pharmaceutical agents with ostensibly equivalent bioavailability profiles constitutes a non-trivial risk to patient homeostasis, particularly in the context of medications possessing a narrow therapeutic index. The regulatory framework, while ostensibly rigorous, fails to account for inter-individual pharmacokinetic variance, rendering blanket equivalency ratings statistically misleading. One must therefore exercise prudence, and in the interest of clinical integrity, advocate for continuity of formulation whenever possible.
In India, we use generics for everything. But for NTI drugs? We don't mess around. If you're on tacrolimus after transplant? You get the same brand. Same batch. Same pharmacist. We know what happens when you play Russian roulette with your immune system. My uncle's liver? Gone because they switched generics without telling him. Now we say: if it works, don't touch it. No drama. No cost talk. Just life.
I switched to generic carbamazepine last year and had zero issues. But I also got my levels checked every 2 weeks for the first month. I didn't assume. I monitored. And I talked to my neurologist. It's not about fear. It's about being an active participant in your care. You can save money and stay safe. It's not either/or.
Oh my god. I just realized I've been on three different generic levothyroxine brands in the last 18 months. My TSH went from 2.5 to 7.8 to 1.2 to 5.1. I thought I was going crazy. Turns out my pharmacy kept switching me. I had to call my doctor and demand a "dispense as written" on my script. Now I have a sticky note on my fridge that says "NO SUBSTITUTIONS". And I make sure the pharmacist sees it every time. This isn't about money. It's about your life. And if you're not fighting for your meds, who will?
Let me be clear. The fact that we even have to discuss this is a failure of American healthcare. In Germany, they don't mess with NTI generics. In Japan, they don't. But here? We treat life-saving drugs like grocery store cereal. Buy the cheapest one. No one checks the ingredients. No one cares if the body rejects it. This isn't capitalism. This is negligence dressed up as savings. And until we stop treating human biology like a cost center, people will keep dying because someone in a corporate office decided a 40-cent difference matters more than a 40-year life.