Most people assume that a generic pill is just a cheaper version of the brand-name drug-same active ingredient, same effect. And for the vast majority, that’s true. But when your body reacts differently after a switch-new headaches, weird rashes, or sudden anxiety-it’s not just in your head. Something real is happening. You’re not imagining it. And you might need to act fast.
Why Your Body Reacts Differently to Generic Drugs
Generic drugs are required to have the same active ingredient as the brand-name version. That part is non-negotiable. But everything else? That’s where things get messy. The fillers, dyes, coatings, and preservatives-called excipients-are not regulated to be identical. And for some people, those tiny differences make a huge difference. Take levothyroxine, the common thyroid medication. One manufacturer uses lactose as a filler. Another uses cornstarch. If you’re lactose intolerant, even a small amount can throw off your thyroid levels, making you feel tired, shaky, or heart-racing-even though the active ingredient is exactly the same. A 2023 GoodRx analysis found that 23.7% of patients switching to generic levothyroxine reported new or worse symptoms. That’s more than one in four. The same goes for psychiatric meds. A Reddit user named u/ADHDstruggles reported that generic Adderall from one company gave him stomach cramps and headaches, while another generic version worked fine. The brand-name version? No issues at all. Why? Different binders. Different coatings. Different manufacturing environments. Even the size of the particles in the pill can change how fast the drug is absorbed-and that matters, especially for drugs with a narrow therapeutic index.What Is a Narrow Therapeutic Index? (And Why It Matters)
Some drugs don’t have a lot of room for error. A tiny change in blood levels can mean the difference between treatment and danger. These are called narrow therapeutic index (NTID) drugs. The FDA lists 18 of them. Among the most common:- Warfarin (Coumadin) - a blood thinner
- Levothyroxine (Synthroid) - for thyroid function
- Phenytoin (Dilantin) - for seizures
- Lithium - for bipolar disorder
Excipients: The Hidden Culprits
You won’t find excipients listed on the pill bottle in plain language. But they’re there. And they’re not harmless. - Gluten - found in some generic versions of seizure meds and antidepressants. Can trigger gut damage in celiac patients, even if they’ve never had symptoms before. - Lactose - used as a filler in hundreds of generics. Can cause bloating, diarrhea, or even mimic hypothyroid symptoms. - Artificial dyes - red 40, yellow 5, blue 1. Linked to headaches, rashes, and behavioral changes in kids. Found in liquid versions of ADHD meds and children’s antibiotics. - Benzalkonium chloride (BAK) - a preservative in generic eye drops. The branded version, Travatan Z, uses a different preservative. Patients switching to the generic often report burning, redness, and vision blur. One ophthalmology study found 68% of patients with prior sensitivity improved after switching back to the brand. - Saccharin and peppermint - used in chewable or flavored tablets. Can irritate the gut lining, causing nausea or acid reflux. These aren’t rare. They’re standard. And if you’ve got a sensitivity, your body knows.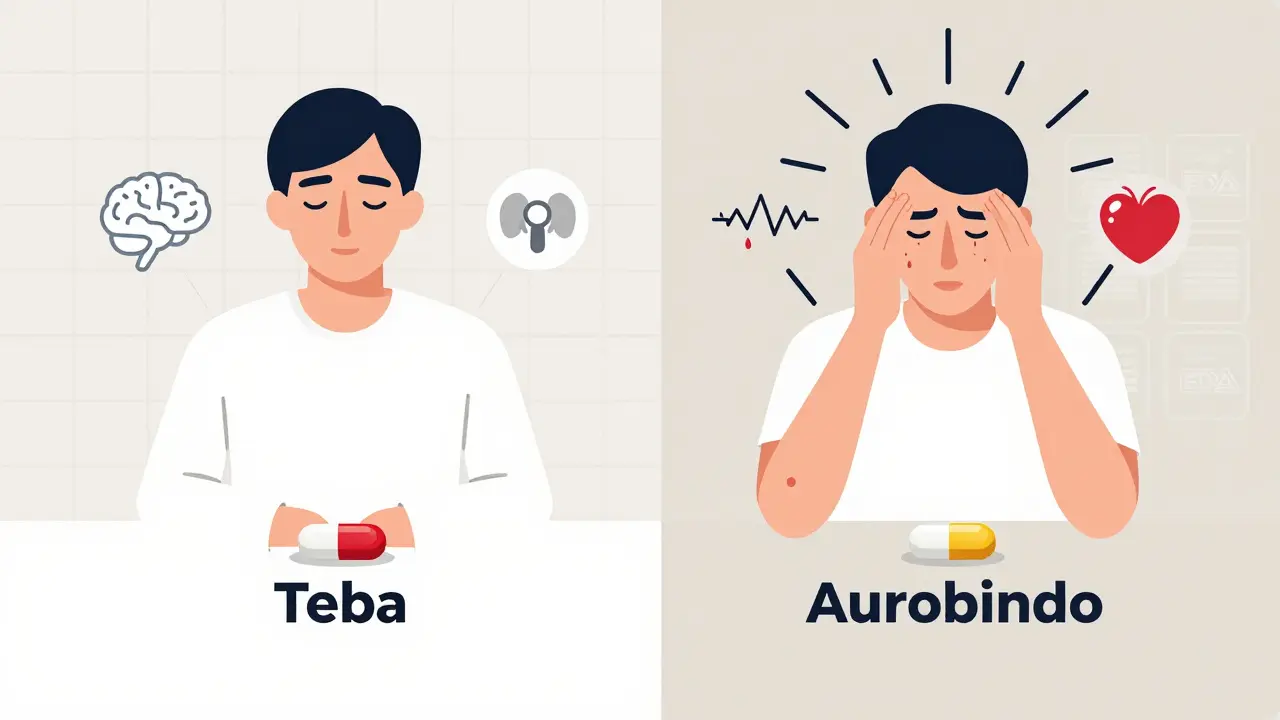
When to Seek Help-Red Flags You Can’t Ignore
Not every new symptom means you need to rush to the ER. But some signs are non-negotiable. If you experience any of these within 1-2 weeks of switching to a generic, contact your doctor immediately:- Severe skin reaction - rash that spreads, blisters, peeling skin, or sores in your mouth or eyes. This could be Stevens-Johnson Syndrome, a rare but life-threatening reaction linked to lamotrigine, allopurinol, and some antibiotics.
- Priapism - a painful erection lasting more than 4 hours. Seen with trazodone, bupropion, and some ED meds. Requires emergency treatment to prevent permanent damage.
- Unexplained bleeding or bruising - especially if you’re on warfarin or another blood thinner. Check your gums, stools, or urine.
- Sudden confusion, seizures, or loss of coordination - could mean your seizure meds aren’t being absorbed properly.
- Heart palpitations, chest pain, or dizziness - especially if you’re on blood pressure or thyroid meds.
- Severe nausea or vomiting lasting more than 48 hours - could signal a reaction to a new filler or coating.
What to Do If You Suspect a Generic Is Causing Problems
1. Don’t stop the medication cold. Stopping thyroid meds, seizure drugs, or blood thinners suddenly can be dangerous. Talk to your provider first. 2. Check the manufacturer. Look at the pill. It’s often printed on the tablet. Or check the pharmacy label. Some generics are labeled with the manufacturer’s name (like “Aurobindo” or “Teva”). Keep a note of which one works and which one doesn’t. 3. Ask your pharmacist to hold the same manufacturer. Pharmacists can often fill your prescription with the same generic brand if you request it. You might have to pay a little more, but it’s worth it if your body responds better. 4. Ask your doctor to write “Dispense as Written” or “Do Not Substitute” on the prescription. This legally prevents the pharmacy from switching your generic without your doctor’s approval. 5. Report it to the FDA. Use MedWatch, the FDA’s online system for reporting adverse events. Include the drug name, manufacturer, lot number (found on the bottle), and your symptoms. These reports help the FDA spot dangerous patterns.The Bigger Picture: Why This Keeps Happening
Generic drugs save the U.S. healthcare system over $1.6 trillion a year. That’s huge. But the system is built on volume, not individualization. The top five generic manufacturers now control nearly 44% of the U.S. market. That means fewer options when one version causes problems. Over 70% of the active ingredients in generics are made overseas-in India and China. The FDA inspects only a fraction of these facilities. A 2022 Government Accountability Office report found that 18% of foreign plants had delayed or limited inspections. That’s not a guarantee of safety. It’s a gamble. And here’s the kicker: the FDA’s own data shows that 1,842 adverse events between 2020 and 2023 were directly tied to switching generics. The most common? Gastrointestinal issues, neurological symptoms, and skin reactions. New legislation introduced in February 2024-the Generic Drug Safety Act-would require manufacturers to notify prescribers and pharmacists whenever they change excipients. That’s a step forward. But right now, you’re the first line of defense.What You Can Do Today
- If you’ve been on a generic for months and feel fine? Keep going. Most people are fine. - If you switched recently and feel off? Track your symptoms. Note the date, the manufacturer, and what changed. - If you’re on a high-risk drug (thyroid, seizure, blood thinner, psychiatric), ask your doctor if you should stick with one manufacturer. - Keep a small note in your phone: “My thyroid med works best with Teva. Avoid Aurobindo.” - Don’t be afraid to ask your pharmacist: “Is this the same manufacturer as last time?” Your body knows what it likes. Trust it. And if something feels wrong after a switch? It probably is.Can generic drugs really cause different side effects than brand-name drugs?
Yes. While the active ingredient is identical, the inactive ingredients-like fillers, dyes, and preservatives-can vary between manufacturers. These differences can trigger reactions in sensitive individuals, especially with drugs that have a narrow therapeutic index, like warfarin or levothyroxine. Studies show 8-15% of patients report new or worsening side effects after switching to a generic.
Which generic medications are most likely to cause problems?
Drugs with a narrow therapeutic index carry the highest risk: thyroid meds (levothyroxine), blood thinners (warfarin), seizure meds (phenytoin, lamotrigine), psychiatric drugs (lithium, some antidepressants), and certain heart medications. Also, complex formulations like eye drops, inhalers, and transdermal patches are more likely to have formulation differences that affect absorption.
What should I do if I think my generic drug is making me sick?
Don’t stop taking it without talking to your doctor. Write down your symptoms, when they started, and which manufacturer made your pill. Call your doctor or pharmacist. Ask if you can switch back to the previous version or request a different generic. You can also report the issue to the FDA through MedWatch.
Can I ask my doctor to prescribe only one brand of generic?
Yes. Your doctor can write “Dispense as Written” or “Do Not Substitute” on your prescription. This legally prevents the pharmacy from switching your generic without your doctor’s approval. Some pharmacies will honor this request even without it written-just ask.
Are generic drugs from India or China less safe?
Not necessarily-but oversight is inconsistent. Over 70% of active ingredients in U.S. generics come from India and China. The FDA inspects only a small percentage of these facilities each year. While many are compliant, delays and limited inspections mean quality control can vary. That’s why tracking the manufacturer and reporting side effects matters.






Okay, I need to say this out loud: I switched my levothyroxine to a generic last year and spent three months feeling like a zombie with a side of panic attacks. No one believed me until I tracked the manufacturer-Teva worked, Aurobindo turned me into a trembling mess. I even called the pharmacy and demanded the same batch every time. They thought I was crazy. Turns out, I was just smart. Your body isn't broken. The pill is.
And yes, the dyes? Real. My niece broke out in hives from a generic amoxicillin with Red 40. No one even thought to ask if she was sensitive to food dyes. Now we check every bottle like it's a bomb squad inspection. Don't let them gaslight you into thinking it's 'all in your head.' It's not.
Also, if you're on warfarin? DO NOT SWAP BRANDS. I saw a guy in the ER last month because his INR went from 2.1 to 6.8 after a 'routine' switch. He almost bled out. This isn't theoretical. It's life-or-death math.