Switching from a brand-name respiratory inhaler to a generic version might seem like a simple cost-saving move - but it’s not as straightforward as swapping one pill for another. For people with asthma or COPD, the device itself is just as important as the medicine inside. If you’ve been switched to a new inhaler without warning, and now your symptoms feel worse, you’re not alone. Thousands of patients have reported confusion, worsening breathing, and even emergency room visits after being switched to a generic inhaler with a different design. This isn’t about brand loyalty - it’s about how the device works, and whether you’ve been properly trained to use it.
Why Inhalers Are Different from Pills
When you switch from brand-name ibuprofen to a generic version, you expect the same pain relief. That’s because the active ingredient is chemically identical, and your body absorbs it the same way. But with respiratory combination inhalers - like those containing budesonide and formoterol - the medicine is only half the story. The other half is the device: how you hold it, how hard you breathe in, whether you need to twist, click, or slide to load the dose. These physical differences change how much medicine actually reaches your lungs.
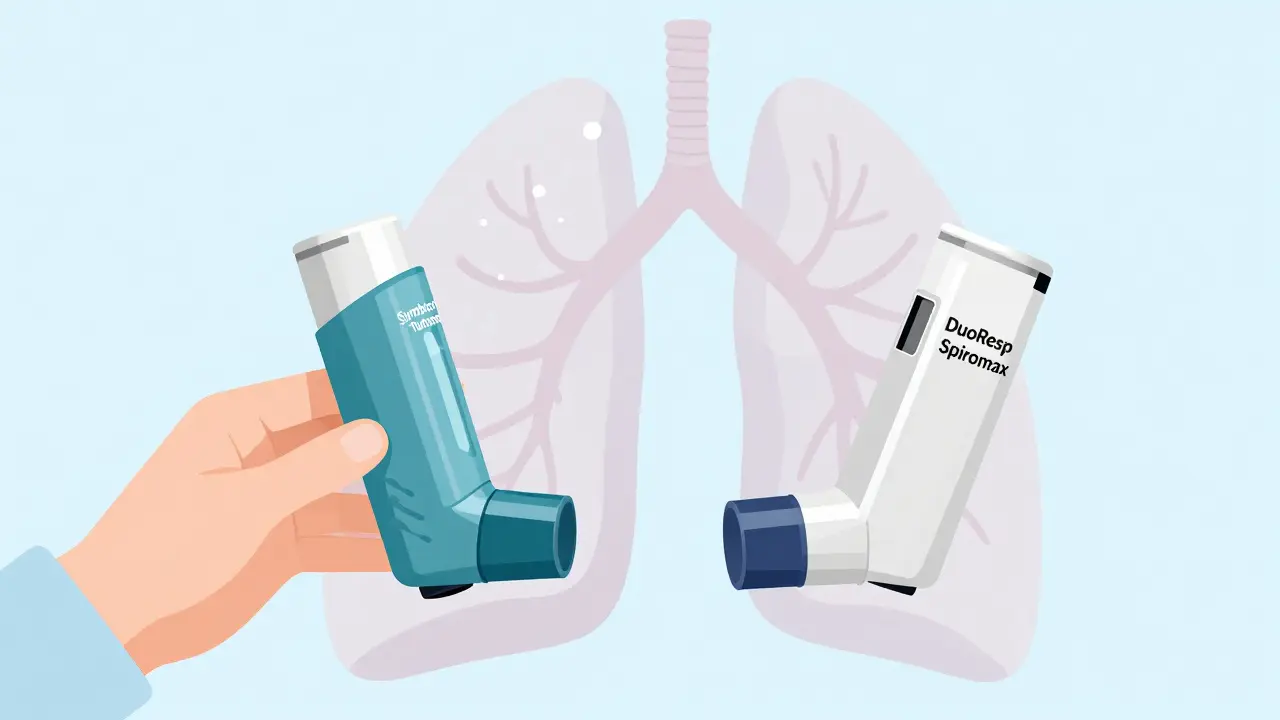
Take the Symbicort Turbohaler and its generic alternative, DuoResp Spiromax. Both contain the same drugs: budesonide and formoterol. But the Turbohaler requires you to twist the base to load a dose. The Spiromax uses a side slider. If you’re used to twisting and suddenly you’re sliding, you might not load the dose properly. And if you don’t load it, you’re not getting the medicine. A 2020 study found that 76% of patients switched without training used the new device incorrectly. That’s not a small risk - it’s a major safety issue.
The Device Matters More Than You Think
There are three main types of inhalers: pressurized metered-dose inhalers (pMDIs), dry powder inhalers (DPIs), and nebulizers. Combination inhalers for asthma and COPD are mostly DPIs. But not all DPIs work the same. Some need a strong, fast breath to pull the powder into your lungs. Others work with a slower, deeper breath. If you’ve been using one type for years and suddenly get a new one that requires a different breathing pattern, you’re at risk.
For example, the Turbuhaler requires a forceful inhalation. The Spiromax needs a similar effort, but the way you activate it is completely different. Patients who were switched without training often breathed too slowly, didn’t inhale hard enough, or forgot to prime the device. The result? Less medicine in the lungs, more side effects in the mouth, and more flare-ups. A 2021 study showed that patients switched from Symbicort Turbohaler to Spiromax without proper instruction had a 22% increase in asthma attacks within six months.
Regulations Vary - and So Do Risks
The rules for approving generic inhalers aren’t the same everywhere. In the U.S., the FDA says if a generic inhaler is approved, patients should be able to use it without extra training. That’s based on the idea that the medicine is the same, so the device shouldn’t matter. But real-world data contradicts that. In Europe, the EMA takes a different view. They require proof that the generic device delivers the same amount of medicine to the lungs as the original. That’s a higher bar - and it’s why fewer generics are approved there.
The UK’s NICE guidelines are blunt: switching inhaler devices without a consultation can lead to worse asthma control. They don’t just recommend caution - they warn against automatic substitution. In the U.S., community pharmacies report that only 28% consistently offer device training when switching inhalers. Time is the biggest barrier. Pharmacists are busy. Training someone on how to use a new inhaler takes 10 to 15 minutes. Most don’t have that time.

What Patients Are Saying
Real people are feeling the impact. On Reddit’s asthma community, 83% of patients who were switched to a generic inhaler without warning reported worse symptoms. A survey by Asthma UK found that 57% of patients felt confused after switching, and 32% ended up in the emergency room within three months. On Drugs.com, Symbicort Turbohaler has a 6.2 out of 10 rating. The generic Spiromax? Only 4.8 out of 10. Common complaints: “Harder to use,” “Feels less effective,” “I didn’t know I had to breathe harder.”
One patient wrote: “My pharmacy switched me to the Spiromax without telling me. I didn’t realize I needed to breathe in harder. My asthma got so bad I ended up in the hospital.” That’s not an outlier. That’s a pattern.
But here’s the good news: when patients get proper training, outcomes improve dramatically. A 2022 study found that 89% of people who received hands-on instruction from their doctor or pharmacist used their new inhaler correctly. That’s the difference between a risky switch and a safe one.
What You Should Do
If your pharmacy or doctor switches your inhaler, don’t assume it’s the same. Ask these questions:
- Is this a different device than what I was using?
- Can you show me how to use it - right now?
- Can I demonstrate it back to you so you know I got it right?
- What should I do if my symptoms get worse after switching?
Use the “teach-back” method. After someone shows you how to use the inhaler, do it yourself. If you can’t do it correctly the first time, ask again. Repeat until you’re confident. Studies show this increases correct use from 35% to 82%.
If your provider refuses to train you, ask for a copy of the device instructions. Most manufacturers have videos online. Watch them. Practice in front of a mirror. Don’t rely on memory.

What Providers Should Do
Doctors and pharmacists need to stop treating inhaler substitution like a pharmacy transaction. It’s a clinical intervention. Every switch should be treated like a new prescription - with education, documentation, and follow-up. In Germany, pharmacists are legally required to give 15 minutes of in-person training for first-time inhaler users. That’s the standard we should aim for.
Health systems that implemented structured inhaler education programs saw a 41% drop in substitution-related emergencies. That’s not just better care - it’s cheaper care. A 2023 report estimated that improper substitution costs the U.S. healthcare system $1.2 billion a year in avoidable ER visits and hospital stays. That’s more than the savings from switching to generics.
The Future Is Here - And It’s Smarter
Some new inhalers now come with built-in sensors that track when you use them and how you breathe. Devices like Propeller Health can detect if you’re inhaling too slowly or not holding your breath long enough. They send alerts to your phone and even to your doctor. A 2022 study in JAMA Internal Medicine found that patients using these smart inhalers had 33% fewer asthma attacks when they were switched to a new device.
Technology like this won’t fix everything - but it can catch mistakes before they lead to emergencies. As more generics enter the market, these tools will become essential. The GINA 2023 guidelines now say: “Device familiarity and correct technique should be prioritized over generic substitution.” That’s a major shift. Cost matters. But not if it costs you your breathing.
Bottom Line
Generic inhalers aren’t inherently bad. They can save money. But they’re not interchangeable like pills. The device is part of the medicine. If you’re switched without training, you’re being put at risk. Demand to see how it works. Practice until you’re sure. If your provider won’t help, find someone who will - a respiratory therapist, a pharmacist with time, even a YouTube video from a reputable source.
Your lungs don’t care about the label. They care about whether the medicine reaches them. Don’t let a switch cost you your breath.
Can I just switch to a generic inhaler without asking my doctor?
No. Generic inhalers may have the same active ingredients, but the device often works differently. Switching without training increases your risk of using it incorrectly, which can lead to worse symptoms, more flare-ups, or even hospital visits. Always ask your doctor or pharmacist before switching - and insist on a hands-on demonstration.
Why do some people say their generic inhaler doesn’t work as well?
It’s not usually that the medicine is weaker - it’s that the device requires a different technique. If you’re used to twisting a Turbohaler and now you’re sliding a Spiromax, you might not load the dose properly. Or if you’re used to a slow breath and the new device needs a fast, forceful one, you won’t get the full dose. This leads to less medicine in your lungs, which feels like the drug isn’t working - even though it’s your technique that’s off.
Are all generic inhalers the same?
No. Even two generics for the same drug can use different devices. One might be a slider, another a twist, and another a button-press. Each requires a different breathing pattern and hand motion. Just because two inhalers contain budesonide and formoterol doesn’t mean they’re interchangeable. Always check the device name - not just the drug name.
What should I do if I’ve already been switched and my breathing is worse?
Don’t ignore it. Contact your doctor or respiratory therapist immediately. Bring your new inhaler with you. Ask them to watch you use it and tell you if you’re doing it right. You may need to switch back to your original device or get proper training on the new one. Worsening symptoms after a switch are not normal - they’re a red flag.
Is there a way to tell if I’m using my inhaler correctly?
Yes. The teach-back method works: after someone shows you how to use it, do it yourself. If you’re unsure, record yourself with your phone and compare it to an official video from the manufacturer or a trusted source like the American Lung Association. Look for signs: Did you inhale deeply? Did you hold your breath for 5-10 seconds? Did you rinse your mouth afterward? If you’re missing steps, you’re not getting the full benefit.





This hit home hard. I got switched to a generic last year and thought I was just being lazy about my breathing. Turns out I wasn’t loading the dose right at all. Took me three weeks and a trip to the ER to figure out it wasn’t me-it was the damn device. Now I always ask for a demo. No excuses.
As a pulmonology nurse practitioner, I cannot emphasize enough: inhaler technique is not intuitive. The FDA’s equivalence standards for generics are based on pharmacokinetics, not device ergonomics or patient learning curves. This is a systemic failure in patient education, not a flaw in generics themselves. Training must be standardized, documented, and reimbursable.
lol i just thought i was getting worse at breathing. turned out i was just using the new one like the old one. my pharmacist didnt even say it was different. now i watch youtube videos before i refill. #inhalerfail
My father was switched without warning. He’s 72, has COPD, and has used the same inhaler for 12 years. The new one felt alien to him. He stopped using it for two weeks because he was afraid he was doing it wrong. By the time he got help, his oxygen levels were dangerously low. This isn’t about cost. It’s about dignity and safety.
One must consider the epistemology of medical substitution. The assumption that chemical equivalence implies therapeutic equivalence is a reductionist fallacy. The inhaler is not merely a delivery mechanism-it is a prosthetic extension of the patient’s respiratory autonomy. When the interface changes without consent or instruction, the patient’s agency is violated. This is not pharmaceutical innovation; it is administrative negligence disguised as efficiency.
they switch you to a generic like it’s a coupon for a new flavor of yogurt. ‘Oh, this one’s cheaper!’ yeah, and now my lungs are yelling at me. i asked my doc why i’m coughing more and she said ‘maybe you’re just getting older.’ thanks, doctor. i’ll just go die quietly.
They’re doing this on purpose. Big Pharma wants you to fail so you’ll go back to the brand name. The FDA is in their pocket. I saw a guy on TikTok say the generic inhalers have a microchip that tracks you. I don’t trust any of it. My cousin’s neighbor’s cousin got hospitalized after switching. Coincidence? I think not.
Let’s zoom out. The entire healthcare system is built on transactional efficiency-minimize time, maximize throughput, reduce labor costs. But respiratory care is fundamentally relational. It requires tactile demonstration, iterative feedback, and emotional reassurance. You can’t outsource breath to a pharmacy counter. When we treat inhalers like aspirin, we’re not saving money-we’re externalizing the cost onto patients’ lungs, their ER visits, their lost workdays, their fear. This isn’t economics. It’s bioethics in slow motion.
Per the American College of Chest Physicians Clinical Practice Guideline 2023, standardized inhaler training protocols reduce exacerbation rates by up to 48% in high-risk populations. Institutions implementing mandatory device demonstration and competency verification have demonstrated statistically significant improvements in adherence and clinical outcomes. We urge all providers to adopt this standard immediately.
People like you are why we’re all dying. You think it’s ‘just a device’? No. It’s a trap. They swap your inhaler, you mess up, you get sick, then they sell you a new one with sensors and charge you $500. It’s all a scheme. I stopped taking mine entirely. My body’s smarter than the system.
From a pharmacokinetic standpoint, the aerosol particle size distribution and fine particle fraction of generic DPIs are often bioequivalent to originators-but the aerodynamic delivery profile is heavily influenced by patient inhalation flow rate and device actuation timing. The clinical disconnect arises because regulatory endpoints do not mandate real-world usage validation. This is a gap in post-marketing surveillance, not product failure.
generic inhalers have a secret code in the plastic that makes your lungs forget how to work right. i found out because my dog started barking at it. my pharma rep said its ‘normal’ but i know they’re lying. the gov’t is hiding the truth. i’ve been recording my breath with my phone and the waveform looks weird after switching. someone please help.