Aug 26, 2025
Rifaximin and Weight Loss: Does It Work? Evidence in 2025
Archive: 2026/02

Cmax and AUC in Bioequivalence: What Peak Concentration and Total Exposure Really Mean
Read More
Cmax and AUC are the two key pharmacokinetic measures used to prove generic drugs work like brand-name versions. Cmax shows peak concentration, AUC shows total exposure-both must fall within 80%-125% for approval.
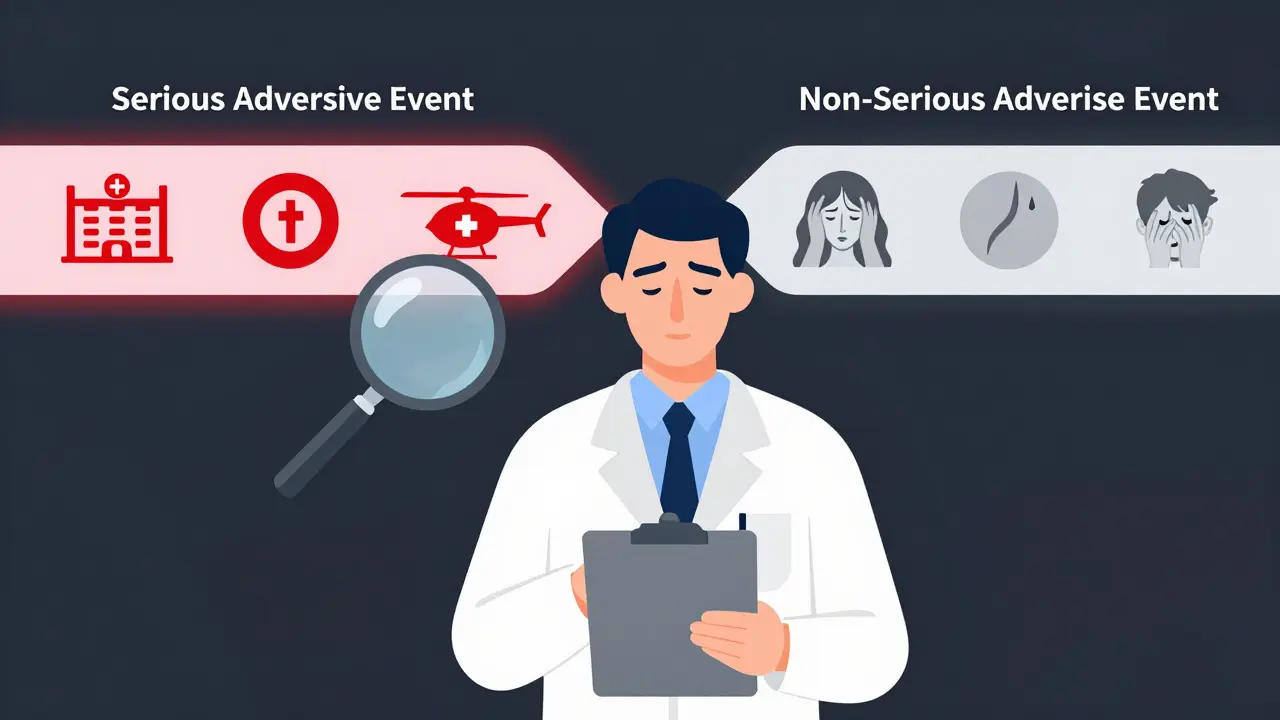
Serious vs Non-Serious Adverse Events: When to Report in Clinical Trials
Read More
Learn the critical difference between serious and non-serious adverse events in clinical trials, when to report each, and how misclassification wastes time and endangers patients. Based on FDA, ICH, and NIH guidelines.

Therapeutic Failures: When a Generic Drug Doesn't Work as Expected
Read More
Generic drugs are supposed to be safe and effective alternatives to brand-name medications, but when they fail to work as expected, the consequences can be serious. Learn why some generics don't deliver the same results-and what you can do about it.