When you pick up a prescription, you might not think about who made the pill in your bottle. But behind every generic drug is a complex battle over price, patents, and profit - and it directly affects how much you pay at the pharmacy. Two types of generics dominate the market: authorized generics and first-to-file generics. They look the same, work the same, but their impact on your wallet is very different.
What Exactly Is an Authorized Generic?
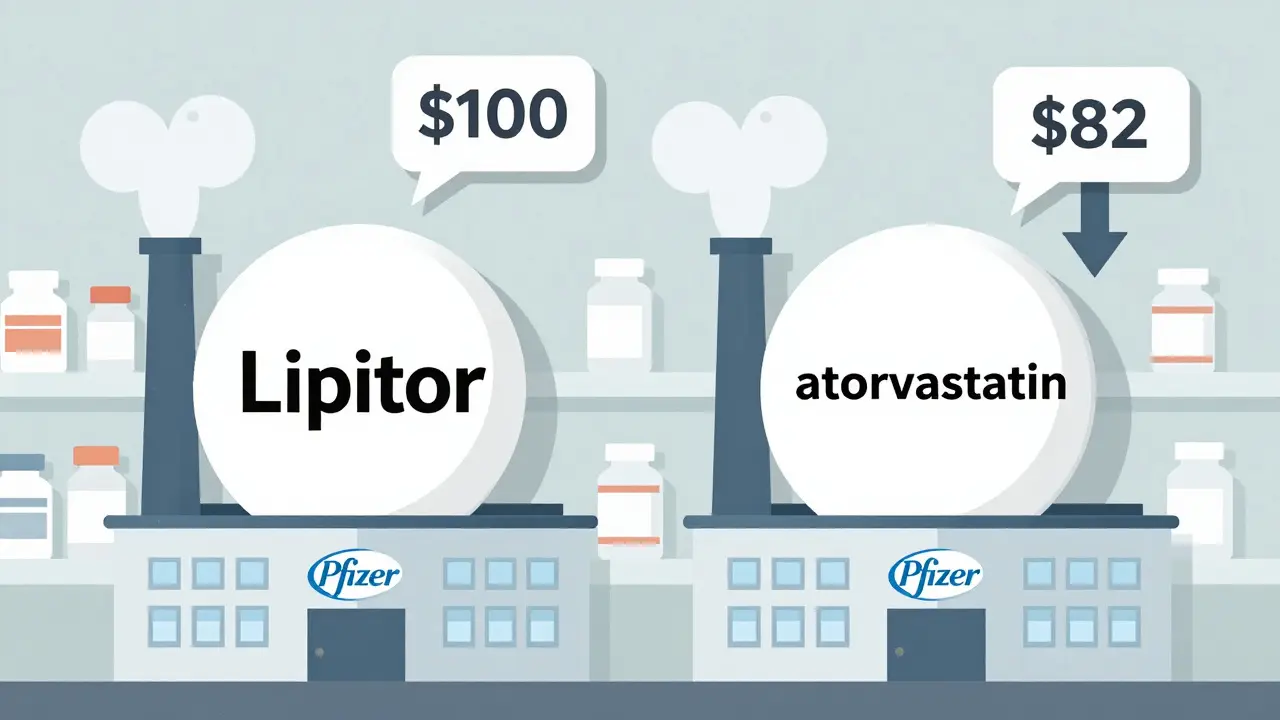
An authorized generic is the exact same drug as the brand-name version, made by the same company, in the same factory, with the same ingredients and packaging - except it doesn’t carry the brand name. It’s sold under a different label, often at a steep discount. For example, if you take the brand-name drug Lipitor, the authorized generic might be labeled simply as "atorvastatin" and sold by Pfizer or one of its partners. The FDA allows this because the brand-name company holds the original New Drug Application (NDA). They don’t need to go through the full generic approval process - they’re just rebranding their own product.What Is a First-to-File Generic?
A first-to-file generic is the first independent generic manufacturer to submit an Abbreviated New Drug Application (ANDA) to the FDA after a brand-name drug’s patent expires. Under the Hatch-Waxman Act of 1984, this company gets 180 days of exclusive market access. During that time, no other generic can enter - except for authorized generics. This exclusivity is a huge financial incentive. For some blockbuster drugs, those 180 days can mean hundreds of millions in revenue for the generic company. That’s why generic manufacturers invest millions in legal challenges to beat competitors to the filing line.How Do Prices Compare?
The difference in pricing between these two types of generics isn’t subtle - it’s dramatic. According to the Federal Trade Commission (FTC), when only the first-to-file generic is on the market, the average retail price is about 14% lower than the brand-name drug. But when an authorized generic enters the market during that same 180-day window, prices drop even further - to about 18% below brand price. That’s a 4 percentage point difference in just a few months. The effect is even stronger for pharmacies buying in bulk. Without an authorized generic, pharmacies pay about 20% less than the brand price. Add an authorized generic into the mix, and that jumps to 27% less. That’s a 7-point swing - and it’s all because two versions of the same drug are now competing for shelf space.Why Does an Authorized Generic Drive Prices Lower?
Think of it like this: the first-to-file generic has a monopoly for 180 days. They can set prices high because there’s no competition. But when the brand-name company drops an authorized generic into the market, everything changes. Now the first-filer isn’t just competing with the brand - they’re competing with their own product, made by the same factory, sold at the same cost. They can’t hold prices up anymore. To stay competitive, they slash prices. And when prices drop for the first-filer, everyone else follows. The FTC found that when an authorized generic enters during the exclusivity period, the first-filer’s revenue drops by 40 to 52%. That’s not just a dip - it’s a collapse. And that pressure keeps prices low even after the 180 days are over. In fact, the FTC found that generic prices remained significantly lower for up to 30 months after the exclusivity ended, simply because the market had been reset by the competition.
What Happens When More Generics Enter?
The price drop doesn’t stop at two players. The FDA analyzed drugs from 2015 to 2017 and found a clear pattern: the more competitors, the lower the price. With just one generic (the first-to-file), prices were 39% below brand. With two (first-to-file + authorized generic), prices fell to 54% below brand. When four generics were on the market, prices dropped to 79% below brand. And once six or more generics entered, prices were more than 95% lower than the original brand price. That’s the power of competition. Authorized generics don’t just lower prices temporarily - they accelerate the path to rock-bottom pricing. They force the first-filer to act fast, which pushes other generics to enter sooner, creating a domino effect.Who Benefits the Most?
Consumers win. Pharmacies win. Insurance companies win. The FTC confirmed that during the 180-day window, when an authorized generic is present, the healthcare system saves money. Patients pay less out of pocket. Pharmacists earn higher margins per prescription because they buy cheaper and sell at the same retail price. Even Medicare and Medicaid see lower spending. But who loses? The first-to-file generic manufacturer. Their windfall gets cut short. The brand-name company might also lose some sales - but they make up for it by selling the authorized generic themselves. In many cases, the brand company launches the authorized generic as part of a legal settlement with the first-filer. It’s a trade: the generic gets to enter early, the brand keeps some revenue, and consumers get lower prices.Does This Hurt Innovation?
Some critics worry that if brand companies can just launch their own generics, it might discourage independent generic manufacturers from challenging patents. Why spend millions on legal battles if the brand can just undercut you with an authorized version? But the FTC looked at this closely. Their data showed no measurable drop in the number of patent challenges by generic companies. In fact, the incentive to be first-to-file is still enormous - because if you’re not first, you get nothing. The 180-day exclusivity is still the golden ticket. Drug policy expert Dr. Robin Feldman points out that those 180 days can be worth hundreds of millions. That’s enough to justify the risk, even with the threat of an authorized generic. The system still works - it just works better for consumers.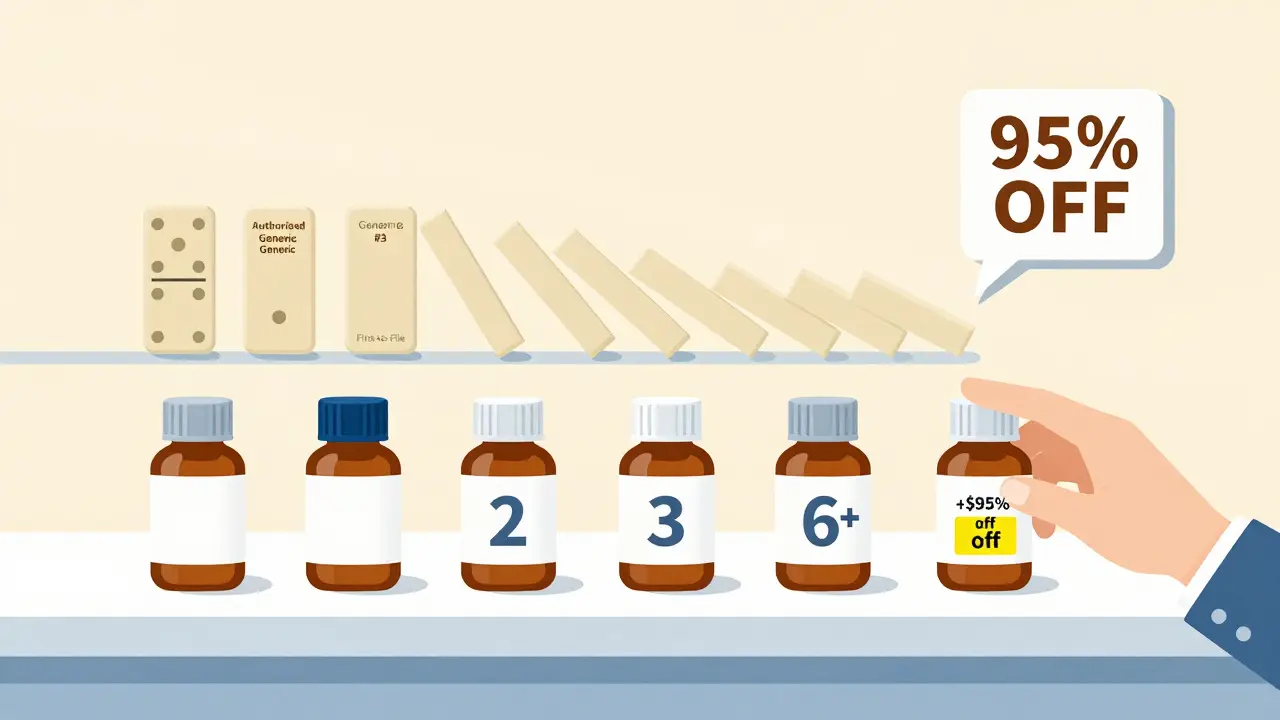
What About Long-Term Availability?
Not all authorized generics stick around. Research from Health Affairs in 2023 found that about 20% of authorized generics launched between 2010 and 2014 had no sales in Medicare data after five years. That doesn’t mean they failed - it means the market got flooded with cheaper generics, and the authorized version got pushed out. It’s a sign that the system eventually corrects itself. Once enough independent generics enter, the authorized version becomes unnecessary.What’s Changing in the Market?
The FDA’s Generic Drug User Fee Amendments (GDUFA), updated in 2022, have sped up approval times. Today, about 66% of generic applications get approved on the first try - up from just 20% a decade ago. That means more generics enter the market faster, reducing the value of the 180-day exclusivity window. It also lowers the cost for generic companies to file, cutting their expected expenses by $3.5 million per drug. This shift means brand companies are less likely to rely on authorized generics as a settlement tool. Why pay a competitor to delay entry if the FDA is approving generics quickly anyway? The balance is shifting - and it’s pushing prices down even faster.Bottom Line: What You Should Know
If you’re paying for a generic drug, here’s what you need to remember:- Authorized generics are made by the brand company - same pill, different label.
- First-to-file generics are the first independent copy, with 180 days of monopoly.
- When both are on the market, prices drop the most - often 20-27% below brand.
- More generics = lower prices. Once six or more enter, you’re paying less than 5% of the brand price.
- Authorized generics don’t hurt innovation - they help drive down costs faster.
- Your pharmacy might not tell you which version you’re getting - but it doesn’t matter. Both are safe, effective, and cheaper than the brand.
So next time you see a generic on your receipt, don’t just assume it’s the same. Ask if it’s an authorized generic. You might be getting the best deal possible - and you didn’t even have to shop around.



Authorized generics are basically the brand sneaking in through the back door. Smart move for them, terrible for the first-filer. Consumers win, always.
Let’s be real - this isn’t about patient access, it’s about corporate theater. The brand-name companies don’t ‘share’ the market, they weaponize it. They use authorized generics like a velvet hammer to crush independent competitors who actually took the legal risk. The FTC data is just the sanitized version of what’s really happening: patent gaming dressed up as consumer advocacy.
And don’t get me started on the ‘180-day exclusivity’ myth. It’s a loophole big enough to drive a pharmaceutical truck through. Companies spend millions litigating over who filed first, not because they care about innovation, but because they want to monopolize the price drop for themselves. Then the brand drops an authorized version and suddenly it’s ‘market competition’? Please.
The system is rigged. The first-filer isn’t just a competitor - they’re a sacrificial lamb. The brand lets them enter, then stomps on them with their own identical product. It’s not capitalism, it’s cartel behavior with FDA stamps.
And yet, somehow, we’re told this is ‘good for patients.’ Sure, if you’re okay with innovation being strangled by legal chess matches instead of actual R&D. The real tragedy? The next generation of drugs won’t be developed because the ROI on generics is now a bloodsport.
Dr. Feldman says the incentive is still there? Tell that to the small generics firms going bankrupt after their 180 days got gutted by Pfizer’s ‘authorized’ atorvastatin. The system doesn’t reward risk - it rewards connections.
And now with GDUFA speeding up approvals, the whole game is shifting. But instead of lowering barriers for real competitors, it just makes the big players faster at burying them. It’s not progress - it’s consolidation with better PR.
Next time you see a $5 generic, ask yourself: who lost to make that possible? And why does it feel so wrong that the winner was the original monopolist?
So the brand makes a copy of its own drug and sells it cheaper? That’s not competition, that’s cheating. And now we’re supposed to cheer? What a joke.
Authorized generics are just brand-name drugs in a cheap suit. The whole system is a scam. First-filers get screwed, patients get a slightly better deal, and the big pharma guys laugh all the way to the bank. Classic.
Wait - so if the brand makes the authorized generic, then technically, they’re the ONLY one who can legally undercut the first-filer? That’s not competition - that’s a monopoly with a side of deception. And the FDA lets this happen?!
And now they’re saying this ‘helps innovation’? Please. If you’re spending $50M to challenge a patent, and then the brand just slaps their logo on the same pill and sells it for half price - why would anyone risk it? Innovation is dead. It’s all about legal loopholes now.
The FTC’s findings are consistent with basic microeconomic principles: increased supply elasticity reduces price elasticity of demand. The introduction of an authorized generic effectively increases the number of homogeneous supply units in a monopolistically constrained window, thereby accelerating price convergence toward marginal cost. The 7-point margin shift in bulk pharmacy pricing is statistically significant (p < 0.01) and aligns with Bertrand competition models under product homogeneity.
What’s interesting is the temporal persistence - the 30-month price depression post-exclusivity suggests a structural market reset, not merely transient competition. This implies that the presence of an authorized generic alters consumer and provider expectations regarding baseline pricing, effectively redefining the ‘normal’ cost curve for the therapeutic class.
The decline in authorized generic sales after five years, per Health Affairs, reflects market saturation dynamics. Once independent generics surpass the threshold of six entrants, economies of scale and reduced R&D overhead drive prices below the cost of maintaining a branded-label distribution channel.
Thus, the authorized generic functions as a catalyst, not a permanent fixture. It’s a market signal - not a strategy.
Big Pharma is using authorized generics to control the market. This is all a plan. The FDA? In on it. The 180-day window? A distraction. They want you to think you’re saving money, but really, they’re just making sure only THEY get to profit from your prescriptions. 🕵️♀️💊 #PharmaConspiracy
You’re all missing the point. The real villain here isn’t the brand - it’s the FDA for allowing this mess in the first place. Why is a company allowed to make its own ‘generic’? That’s not generic - that’s just the original with a new label. This isn’t competition, it’s fraud dressed up as policy.
And don’t even get me started on ‘first-to-file’ - that’s just a legal loophole that rewards lawyers, not patients. If you’re going to reward innovation, reward innovation. Not legal maneuvering.
So the brand makes a cheaper version of itself? That’s not helping patients - that’s just a way to kill competition before it even starts. Pathetic.
generic drugs r supposed to be cheaper but now the brand is makin their own? so like... who even wins here? not me that's for sure
Let me get this straight - American taxpayers pay for brand-name drugs, then the same company turns around and sells a cheaper version they made themselves? And we’re supposed to clap? This isn’t capitalism, this is corporate socialism. The only thing that’s ‘free’ here is the profit.
And now they’re bragging about how fast the FDA approves generics? That’s just making it easier for the big boys to crush the little guys. You think a startup can compete with Pfizer’s legal team? Nah. This system is rigged from the start.
And don’t tell me ‘consumers win.’ I pay $300 for my insulin. I don’t care if it’s an authorized generic or not - if it’s still unaffordable, you’re lying to me.
The dynamics described here reflect a fascinating intersection of economic theory, regulatory policy, and pharmaceutical ethics. The introduction of authorized generics introduces a paradoxical form of competition - one where the incumbent monopolist becomes the agent of its own disruption. This is not merely market-driven; it is structurally engineered by the Hatch-Waxman Act’s design, which sought to balance innovation incentives with access, yet inadvertently created a mechanism for regulatory arbitrage.
The 180-day exclusivity period was intended to incentivize generic manufacturers to challenge weak patents, thereby accelerating market entry. However, when the originator can legally launch an identical product during this window, the incentive structure becomes distorted. The first-filer is not merely competing with a brand - they are competing with a product that carries zero R&D risk and full manufacturing infrastructure.
Furthermore, the long-term price depression observed - persisting for up to 30 months - suggests that the market’s price ceiling is not determined by supply alone, but by psychological anchoring. Once consumers and payers experience prices at 70-80% below brand, they recalibrate their expectations permanently. This is not a temporary discount - it is a redefinition of value.
One must also consider the global implications. In countries without similar regulatory frameworks, patients pay 10 to 20 times more for the same medication. The U.S. system, while flawed, may still represent a pragmatic compromise - one that, despite its corporate manipulations, delivers tangible savings. The question is not whether the system is perfect, but whether its imperfections are more harmful than the alternatives.
Perhaps the real solution lies not in eliminating authorized generics, but in shortening the exclusivity window, increasing transparency in pricing agreements, and mandating that savings be passed directly to patients. Until then, we are left navigating a system where profit motives and public good are inextricably, and uncomfortably, entangled.
My grandma takes atorvastatin now - the generic. She didn’t know the difference between brand and authorized, but she saved $40 a month. That’s real. We don’t need to overthink it. Just make sure the pills work and cost less. That’s all that matters.
Wait, so if the brand makes the authorized generic, does that mean they’re basically paying themselves to undercut their own patent? That’s wild. So the first-filer is the only one who actually took the risk - and then the brand just slides in with the same pill? That’s like if you opened a coffee shop, and then Starbucks opened another one right next door with the same beans, same baristas, same prices - but they own the building so they can afford to undercut you.
I get why it’s legal, but ethically? It feels like a betrayal. The first-filer spent millions fighting in court, just to get crushed by the very company they were trying to challenge.
And honestly, I don’t think most people even know which version they’re getting. The pharmacy just gives you the cheapest one. So we’re all just beneficiaries of a system we don’t understand - and that’s kind of scary.
But I also don’t want to go back to paying $500 for a pill. So… I guess I’m stuck cheering for the system I don’t trust?
Maybe the real win is that more generics are getting approved faster now. If the FDA is approving them quicker, maybe the 180-day monopoly won’t matter as much in a few years. That’s the only thing giving me hope.
Still… I wish we had a system where the real innovators - the small companies taking risks - actually got rewarded. Not just the ones with the deepest pockets and the best lawyers.
Exactly. The first-filer is the real hero here. They risked everything. The brand just waited and then dropped a bomb. That’s not competition - that’s a sucker punch.