Dec 25, 2025
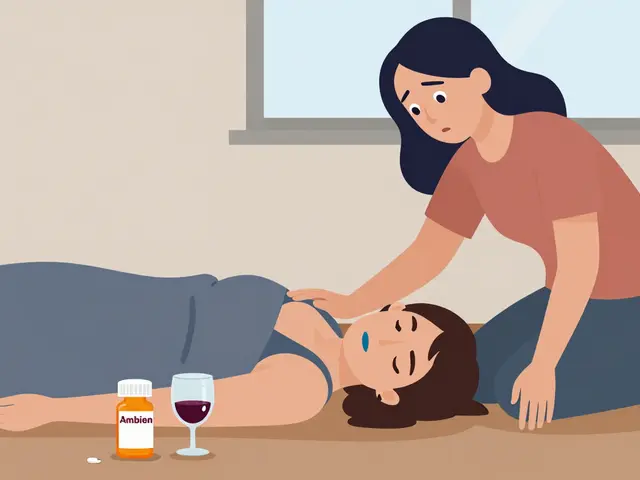
How to Recognize Overdose from Sedatives and Sleep Medications
Tag: pharmacokinetics

Cmax and AUC in Bioequivalence: What Peak Concentration and Total Exposure Really Mean
Read More
Cmax and AUC are the two key pharmacokinetic measures used to prove generic drugs work like brand-name versions. Cmax shows peak concentration, AUC shows total exposure-both must fall within 80%-125% for approval.
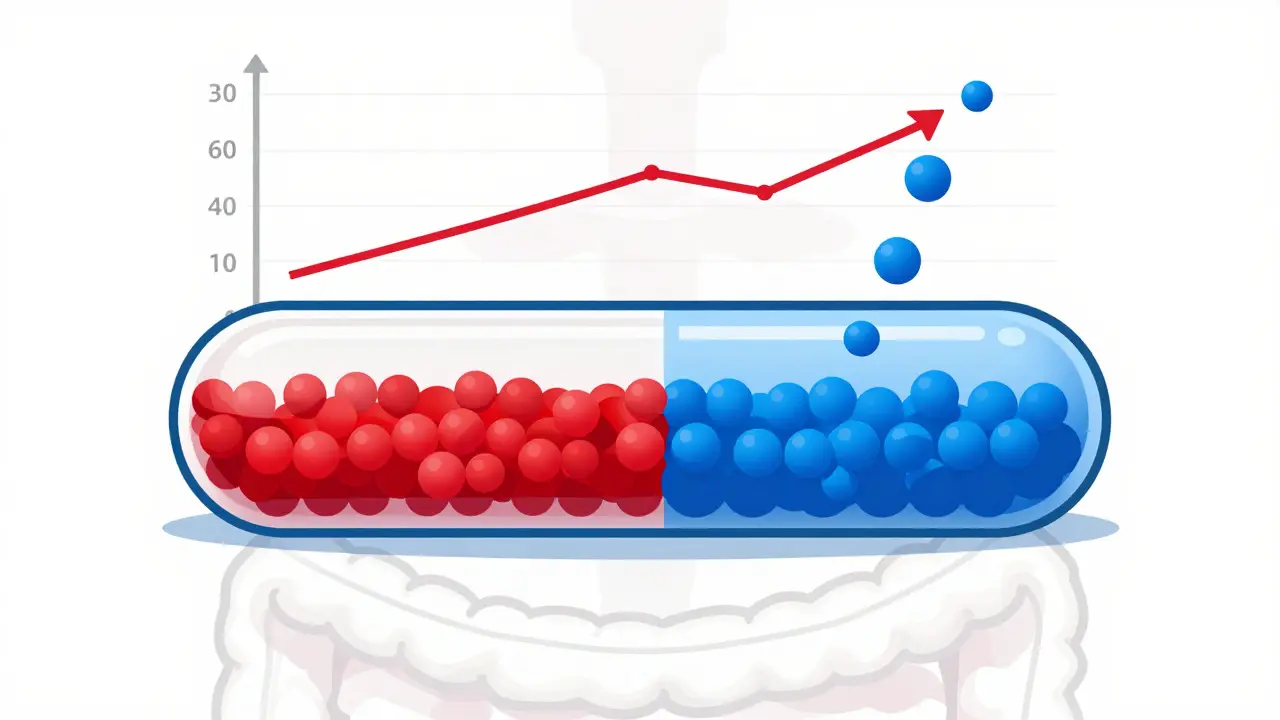
Modified-Release Formulations: Key Bioequivalence Requirements You Need to Know
Read More
Modified-release formulations require special bioequivalence testing to ensure generics work as safely and effectively as brand-name drugs. Learn the key regulatory requirements, common pitfalls, and why standard tests aren't enough.